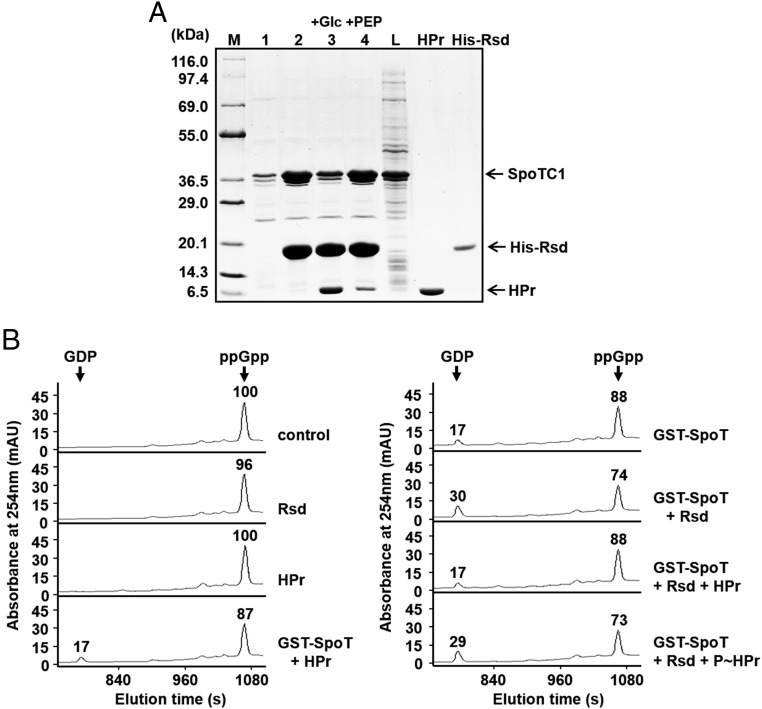
Fig. 3.
The stimulatory effect of Rsd on the SpoT ppGpp hydrolase activity is abolished by dephosphorylated HPr. (A) Interference of the Rsd–SpoT complex formation by a dephosphorylated form of HPr. To test the effect of the phosphorylation state of HPr on the formation of the Rsd–SpoT complex, an E. coli cell extract expressing SpoTC1 was mixed with binding buffer (lane 1), 170 μg His-Rsd (lane 2), or a mixture of 170 μg His-Rsd, 240 μg HPr, and 2 μg EI (lanes 3 and 4), and HPr was dephosphorylated by adding 2 mM glucose (lane 3) or phosphorylated by adding 2 mM PEP (lane 4). Each mixture was incubated with 50 μL TALON resin for metal-affinity chromatography. After elution with imidazole, proteins bound to each TALON resin were analyzed by SDS/PAGE and Coomassie blue staining. Lane M, molecular mass markers; lane L, E. coli cell lysate expressing SpoTC1 before metal-affinity chromatography. Purified HPr and His-Rsd were also run as a control. (B) Stimulation of the SpoT ppGpp hydrolase activity by Rsd is blocked by the dephosphorylated form of HPr. The ppGpp hydrolase activity of purified GST–SpoT (2.5 μg) was assayed in a reaction mixture containing 38.49 μM ppGpp in the presence of different combinations of Rsd (15.6 μg) and HPr (28 μg), as indicated. HPr was phosphorylated by adding EI and 2 mM PEP (P∼HPr). After incubation at 37 °C for 5 min, the reaction mixture was applied to a Supelcosil LC-18-T HPLC column, and ppGpp and GDP were monitored by measuring the A254. Relative peak areas are shown above each peak, considering the peak area of ppGpp in the control sample as 100. Representative data from three independent experiments are shown.