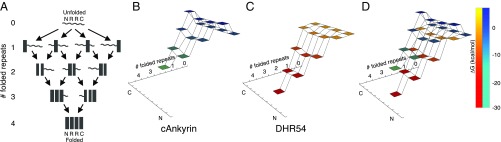
Fig. 4.
Stabilizing intrinsic energies diminish the barriers on folding energy landscapes for DHR proteins in the absence of denaturant. (A) Repeat proteins with NR2C repeat sequences can fold along many pathways. (B–D) Free-energy landscapes from experimentally determined intrinsic and interfacial free energies. The vertical dimension (and shading) shows the free energies of partly folded states along the folding pathway shown in A. (B) Consensus ankyrin repeat proteins, which are based on the naturally occurring ankyrin repeat family, have destabilizing intrinsic energies, and as a result, folding the first repeat results in an early barrier. (C) DHR54 proteins have stabilizing intrinsic folding energies and, as a result, lack this early barrier. Moreover, folding of subsequent repeats is strongly downhill. (D) Overlay of consensus ankyrin (blue–green) and DHR54 (orange–red) free-energy landscapes. Landscapes were generated with Mathematica.