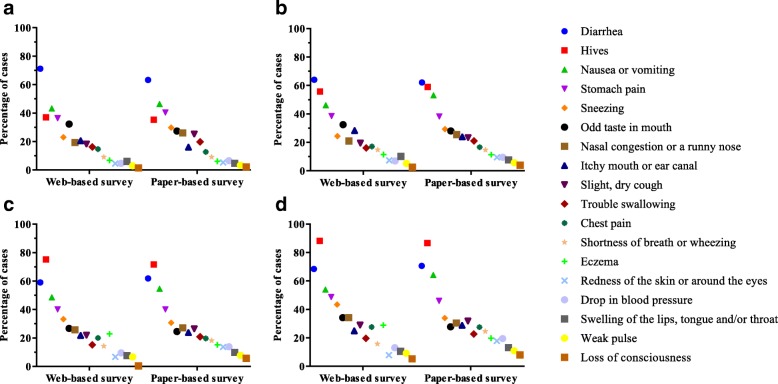
Fig. 2.
Proportion of clinical symptoms reported in two population-based survey modes. a Reported adverse reactions caused by food consumption in the web-based survey (n = 1595) and paper-based survey (n = 6563). b Reported adverse reactions in self-reported FA participant in the web-based survey (n = 515) and paper-based survey (n = 1629). c Reported adverse reactions in doctor-diagnosed FA participants in the web-based survey (n = 105) and paper-based survey (n = 527). d Reported adverse reactions in the IgE-mediated FA group in the web-based survey (n = 91) and paper-based survey (n = 433). The criteria to define IgE-mediated FA include: anaphylaxis reactions (i.e. drop in blood pressure, loss of consciousness, chest pain and weak pulse) or hives/urticaria or angioedema or anaphylaxis reactions after food intake