Abstract
Aims
Echocardiography and tomographic imaging have documented dynamic changes in aortic stenosis (AS) geometry and severity during both the cardiac cycle and stress-induced increases in cardiac output. However, corresponding pressure gradient vs. flow relationships have not been described.
Methods and results
We recruited 16 routine transcatheter aortic valve implantations (TAVI’s) for graded dobutamine infusions both before and after implantation; 0.014″ pressure wires in the aorta and left ventricle (LV) continuously measured the transvalvular pressure gradient (ΔP) while a pulmonary artery catheter regularly assessed cardiac output by thermodilution. Before TAVI, ΔP did not display a consistent relationship with transvalvular flow (Q). Neither linear resistor (median R2 0.16) nor quadratic orifice (median R2 < 0.01) models at rest predicted stress observations; the severely stenotic valve behaved like a combination. The unitless ratio of aortic to left ventricular pressures during systolic ejection under stress conditions correlated best with post-TAVI flow improvement. After TAVI, a highly linear relationship (median R2 0.96) indicated a valid valve resistance.
Conclusion
Pressure loss vs. flow curves offer a fundamental fluid dynamic synthesis for describing aortic valve pathophysiology. Severe AS does not consistently behave like an orifice (as suggested by Gorlin) or a resistor, whereas TAVI devices behave like a pure resistor. During peak dobutamine, the ratio of aortic to left ventricular pressures during systolic ejection provides a ‘fractional flow reserve’ of the aortic valve that closely approximates the complex, changing fluid dynamics. Because resting assessment cannot reliably predict stress haemodynamics, ‘valvular fractional flow’ warrants study to explain exertional symptoms in patients with only moderate AS at rest.
Keywords: Aortic stenosis , Dobutamine , Transcatheter aortic valve implantation
Introduction
Severe aortic stenosis (AS) therapy changed radically with the development and validation of transcatheter aortic valve implantation (TAVI) as an alternative to traditional surgical aortic valve replacement (SAVR). During the 15 years since its initial description,1 TAVI studies have focused on the three interrelated but conceptually separate aspects of any treatment: procedure, patient, and physiology.
First, procedural advances—mechanical, pharmacologic, and imaging—permit the randomized comparison of TAVI vs. SAVR in patients with decreasing surgical risk.2 Second, the development of TAVI-specific risk assessment using clinical characteristics3–5 allows for improved patient selection. Third, several physiologic fluid dynamic descriptions of AS have been proposed, beginning with the orifice model of Gorlin in 1951,6 but with ongoing uncertainty regarding their universal application.7
Pressure loss vs. flow curves describe the fundamental physiology of coronary8 and peripheral9 arterial stenosis. Importantly, echocardiography and tomographic imaging have documented dynamic changes in AS geometry and haemodynamic severity during both the cardiac cycle and stress-induced increases in cardiac output. Current haemodynamic models of AS pathophysiology assume a fixed form. For example, the orifice model predicts a quadratic pressure gradient-flow relation6 while a simple resistor model predicts linear pressure loss across the valve10 as flow increases. The literature documents that the orifice model imperfectly matches the changing aortic valve area (AVA) under stress conditions.11,12 However, systematic characterization of applicable pressure loss vs. flow curves and their implications for AS have not been reported but are especially relevant to TAVI given conflicting severity ratings between AVA and haemodynamics in some cases.
Therefore, our haemodynamic physiology study of AS sought to answer three key questions using valvular pressure loss vs. flow curves. What mix of an orifice or resistor model correctly describes the observed pressure/flow haemodynamics of a stenotic aortic valve? Can we reliably use a resting assessment to predict responses to stress conditions? And how does the pressure gradient vs. flow relationship change immediately after a TAVI procedure?
Methods
Between February and October 2016, we recruited subjects with severe AS from patients undergoing clinical TAVI for standard indications. Inclusion criteria demanded use of general anaesthesia, implantation of an Edwards SAPIEN 3 or Medtronic CoreValve Evolut valve, and native AS (no valve-in-valve). Exclusion criteria included moderate or severe aortic insufficiency, mitral regurgitation, or tricuspid insufficiency; hypertrophic cardiomyopathy; unrevascularized, severe coronary disease; myocardial infarction within the preceding 3 weeks; history of significant ventricular arrhythmia; or a prior severe reaction to dobutamine. All subjects gave written informed consent as approved by the medical ethics committee of the hospital.
Valvular stress physiology protocol
The TAVI procedure initially proceeded as routine for our centre at the time of enrollment, including general anaesthesia, transoesophageal echocardiography, femoral arterial access for a pigtail catheter, femoral venous access for a temporary pacemaker (if the patient did not have a permanent device already), and a second femoral arterial access (typically surgical) for valve implantation. For study purposes, we obtained dedicated venous access (femoral or internal jugular) for a 7F pulmonary artery (Swan-Ganz) catheter with thermodilution capability.
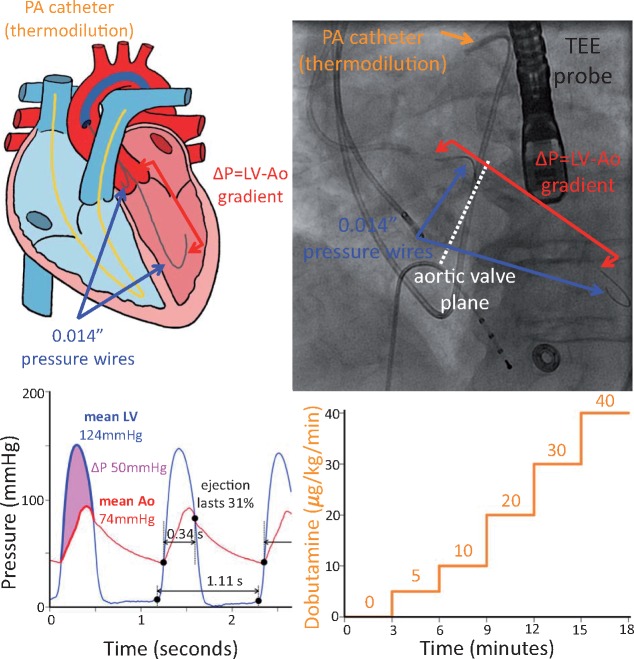
A 6F Amplatz left catheter was negotiated into the left ventricle (LV) using a standard retrograde technique to cross the stenotic aortic valve. Once the catheter was in a stable position, the straight wire was removed and two commercial coronary pressure wires (Aeris PressureWire, St. Jude Medical) were zeroed, inserted, phase aligned, and equalized using a recording system (QUANTIEN analyzer with external pressure wire receiver plus additional Wi-Box, St. Jude Medical) as for coronary physiology. Finally, the catheter and one of the wires were pulled back to the high ascending aorta, minimizing the potential effects of pressure recovery.13 The two 0.014″ wires provided continuous, high fidelity pressure signals in the aorta and LV without imposing an iatrogenic stenosis, as would be the case for a larger, fluid-filled catheter.14Figure 1 provides a conceptual and annotated radiographic view of the protocol.
Figure 1.
Protocol set-up. After routine transcatheter aortic valve implantation preparation, we placed 0.014″ commercial pressure wires in the ascending aorta and across the aortic valve (dashed white line) in the left ventricle to provide high fidelity and uninterrupted measurements of the transvalvular pressure gradient (ΔP). A standard 7F pulmonary artery (Swan-Ganz) catheter enabled thermodilution assessment of cardiac output, while a transoesophageal echocardiographic probe permitted non-invasive evaluation. The upper panels depict the pictorial and fluoroscopic set-up, while the lower panels display the acquired pressure signals and graded dobutamine infusion. Automated analysis identified the start of each beat as well as the ejection period (large black dots in the lower left panel) to compute mean pressures and gradients (highlighted portions of the first beat) as well as the relative duration of ejection (marked for the second beat).
After setup was complete, a step-wise dobutamine infusion began. Each phase lasted approximately 3–5 min, although adjustments were made for echocardiographic imaging duration and individualized subject response. Potential dobutamine doses were 0 (baseline), 5, 10, 20, 30, and 40 μg/kg/min, all delivered via central venous access. The decision to proceed to the next dobutamine dose was made using an integrative, clinical assessment by discussion among cardiac anaesthesia, interventional cardiology, and research team members regarding several parameters: LV, systemic, and pulmonary pressures; cardiac rhythm, especially the presence and frequency of ventricular extras; and left ventricular function and wall motion via echocardiography, using typical stopping criteria for dobutamine stress testing. At baseline, as well as during each stage of dobutamine, two roughly equally spaced thermodilution cardiac output measurements were made. Due to changing oxygen consumption induced by the dobutamine infusion, Fick assessment would not have been feasible. Details regarding acquisition and analysis of echocardiographic data can be found in the Supplementary material online.
Upon completing the stress physiology protocol, we carried out a routine TAVI. The measurements had no influence on whether or not to proceed, device selection, or implantation technique. After the operator was satisfied with the result, including balloon post-dilatation if necessary, an Amplatz catheter was placed in the LV across the implanted valve. Following the steps described previously, the pressure wires were again positioned and the dobutamine infusion repeated. Finally, the pressure wire in the LV was pulled back into the aorta to the same level as the other wire to check for agreement. All catheters and sheaths were removed and post-procedure care proceeded as usual.
Analysis of invasive physiology data
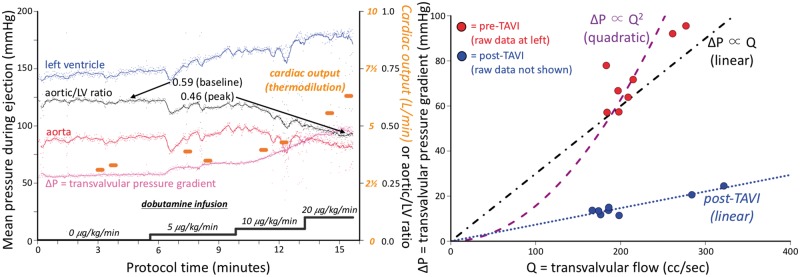
The pressure wires provided measurements every 10 ms to a precision of 0.1 mmHg. Using custom, off-line software, the crossing points of LV and aortic pressure from valid beats were automatically identified. For each valid beat, the analysis software summarized the mean LV and aortic pressures between the aforementioned crossing points (systolic ejection period) as well as its duration relative to the entire cardiac cycle. As a visual summary, we created temporal plots displaying the rate of dobutamine infusion, per-beat and trend line systolic ejection averages of LV, aortic pressure, and average transvalvular pressure loss (ΔP, the mean gradient between LV and aorta), unitless ratio of aortic/left ventricular pressures, and the thermodilution cardiac output (assumed to last a fixed duration of 15 s). Figure 2 (left) depicts a typical example.
Figure 2.
Example haemodynamic data and analysis. The left panel depicts several haemodynamic parameters during the time-course of the protocol. Each small dot represents the systolic ejection portion of a single cardiac cycle, as in Figure1, with a superimposed trend line. Thermodilution cardiac output measurements (orange dots) were made twice during each stage of dobutamine infusion. The right panel demonstrates how each measurement of cardiac output and mean transvalvular gradient (ΔP) was transformed into a single point, creating a pressure loss vs. flow curve. In this example, the shape of the curve before transcatheter aortic valve implantation is neither quadratic (as assumed by the Gorlin orifice model) nor linear (as for a resistor), but instead a mixture of the two. However, after transcatheter aortic valve implantation (raw data not shown but provided in the Supplementary material online and similar in concept to the left panel) the points fall on a straight line through the origin, implying a constant valve resistance.
Using per-beat pressure data combined with thermodilution cardiac output results we created plots of mean transvalvular pressure loss (ΔP) as a function of transvalvular flow (Q). During a 15-s period starting with the bolus of saline for thermodilution, the average systolic ejection transvalvular pressure loss and fraction of the cardiac cycle spent in ejection were computed from valid data. Transvalvular flow represents the cardiac output that passes through the aortic valve and was calculated by dividing cardiac output by the duration of the systolic ejection period relative to the cardiac cycle. For example, a cardiac output of 5 L/min with a relative systolic ejection duration of 33% would produce 5/33% = 15 L/min (or 250 mL/s) of transvalvular flow. Pre-TAVI data for the two resting measurements (before dobutamine infusion) were fit to both linear and quadratic models (ΔP ∝ Qrest and ΔP ∝ Qrest2, where Qrest equals the average, resting transvalvular flow), while all post-TAVI data was fit to a linear model. For each subject both pre- and post-TAVI curves were shown simultaneously as in Figure 2 (right), which displays the ΔP/Q summary of the per-beat data in Figure 2 (left).
Pressure loss vs. flow curves
Using pressure loss vs. flow curves, we developed a conceptual framework for aortic valve physiology based on the notion of changing stenosis geometry, previously applied to coronary lesions.8 For fixed stenosis geometry, the pressure loss vs. flow relationship contains constants describing its viscous and separation components.9 But, if stenosis geometry depends on pressure or flow (as occurs with compliant anatomy subjected to flow-related changes in pressure), then these constants must be replaced by variables. This generalization permits an understanding of the more complex pressure loss vs. flow relationships observed with stenotic aortic valves.
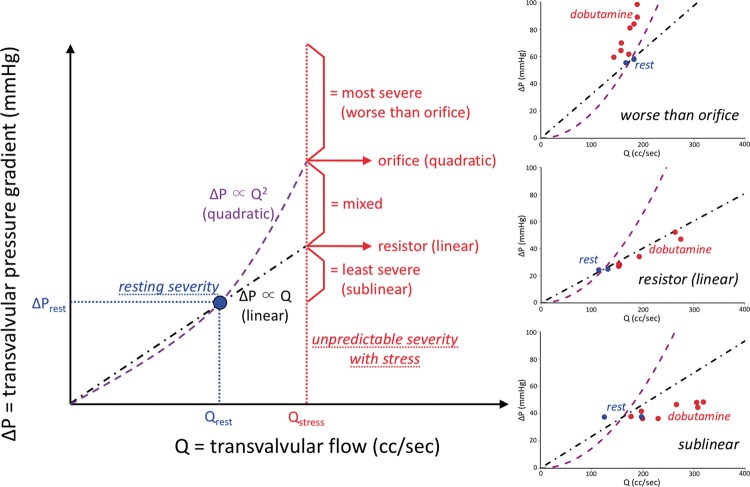
Our new conceptual framework predicted five key patterns of ΔP vs. Q: sublinear (ΔP increases less than predicted by resting measurements due to favourable changes in valvular and outflow tract geometry during stress), linear (valve acts as a pure resistor), mixed (both viscous and separation components), quadratic (pure orifice behaviour as proposed by Gorlin), and superquadratic (ΔP increases even more dramatically than predicted by Gorlin due to worsening stenosis geometry with stress). Full details can be found in the Supplementary material online.
Statistical methods
Analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). We employed standard statistical techniques, and the Supplementary material online provides expanded statistical methods. Applicable tests were two-tailed, and P < 0.05 was considered statistically significant. We did not pre-specify a sample size given the exploratory and descriptive nature of our study, as well as logistical challenges with recruiting subjects from this population and performing the protocol. As detailed in the Supplementary material online, we quantified linear and quadratic model fits using the coefficient of determination (R2), summarized over the cohort by reporting a median value given its non-normal distribution.
Results
We enrolled 16 subjects with baseline and procedural characteristics as summarized in Table 1, reflecting a typical TAVI cohort. All subjects except one had preserved left ventricular function with an ejection fraction >50%, while one subject had a severely reduced ejection fraction <30%. Table 2 displays haemodynamic and physiologic parameters, with mean difference and confidence intervals provided in the Supplementary material online. For technical and clinical reasons, we could not obtain post-TAVI measurements in two subjects (one died during the procedure because of an unexplained cardiac arrest) and in one subject the pre-TAVI cardiac output measurements were technically unsuccessful. The final analysis therefore included 16 subjects before TAVI and 14 subjects after TAVI. The Supplementary material online contains additional results, including plots like Figure 2 for all subjects.
Table 1.
Baseline and procedural characteristics
| Characteristics | Summary (n = 16) |
|---|---|
| Age (years) | 82.3 ± 4.2 |
| Male | 8 (50) |
| Logistic EuroSCORE | 12.3 ± 6.7 |
| Risk factors | |
| Hypertension | 11 (69) |
| Dyslipidaemia | 4 (25) |
| Diabetes mellitus | 6 (38) |
| Active smoking | 1 (6) |
| Major cardiac events | |
| Prior myocardial infarction | 5 (31) |
| Prior PCI | 2 (12) |
| Prior CABG | 4 (25) |
| Cardiac and vascular disease | |
| Cerebral vascular disease | 2 (12) |
| Peripheral vascular disease | 3 (19) |
| COPD | 3 (19) |
| Atrial fibrillation | 9 (56) |
| Permanent pacemaker | 2 (12) |
| Laboratory values | |
| hs-cTnT (ng/L) | 20 (18–28) |
| NT-proBNP (pmol/L) | 165 (84–322) |
| Creatinine (mg/dL) | 0.99 (0.82–1.28) |
| Transcatheter valvea | |
| Medtronic CoreValve Evolut (mm) | 8 (53) |
| 26 | 1 |
| 29 | 7 |
| Edwards SAPIEN 3 (mm) | 7 (47) |
| 23 | 1 |
| 26 | 3 |
| 29 | 3 |
Summary values represent n (%), mean ± standard deviation, or median (IQR).
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; hs-cTnT, high-sensitivity cardiac troponin T; IQR, interquartile range; NT-proBNP, N-terminal pro B-type natriuretic peptide; PCI, percutaneous coronary intervention.
Only 15 valves implanted.
Table 2.
Haemodynamics
| Before TAVI |
After TAVI |
|||
|---|---|---|---|---|
| Baseline | Peak dobutamine | Baseline | Peak dobutamine | |
| Transvalvular systolic gradient (mmHg)a | 45 (40–53) | 67 (53–80) | 9 (5–11) | 13 (8–23) |
| Transvalvular systolic flow (mL/s)a | 162 (143–186) | 270 (198–311) | 224 (163–290) | 340 (282–445) |
| Aortic/LV ratio during systolic ejection (SAVI)a | 0.63 (0.59–0.67) | 0.56 (0.48–0.58) | 0.90 (0.87–0.93) | 0.86 (0.81–0.90) |
| Aortic valve area (cm2)a | 0.54 (0.48–0.59) | 0.65 (0.56–0.89) | 1.78 (1.27–2.24) | 2.31 (1.29–2.97) |
| Cardiac output (L/min)b | 3.2 ± 0.6 | 6.0 ± 2.2 | 3.6 ± 1.0 | 5.9 ± 1.9 |
| Heart rate (b.p.m.)b | 62 ± 14 | 94 ± 21 | 68 ± 12 | 88 ± 23 |
| Stroke volume index (mL/m2)b | 29 ± 7 | 34 ± 11 | 29 ± 11 | 36 ± 11 |
| LV systolic pressure (mmHg)a | 130 ± 23 | 158 ± 26 | 101 ± 25 | 125 ± 33 |
| Aortic systolic pressure (mmHg)c | 83 ± 15 | 89 ± 24 | 92 ± 26 | 109 ± 35 |
| Systolic portion of cardiac cycle (%)d | 33 ± 4 | 37 ± 5 | 30 ± 7 | 29 ± 7 |
Summary values represent mean ± standard deviation or median (IQR).
IQR, interquartile range; LV, left ventricular; SAVI, stress aortic valve index; TAVI, transcatheter aortic valve implantation.
Repeated measures ANOVA P ≤ 0.01 for both before vs. after TAVI and baseline vs. peak dobutamine.
Repeated measures ANOVA P > 0.4 for before vs. after TAVI but P < 0.01 for baseline vs. peak dobutamine.
Repeated measures ANOVA P = 0.029 for before vs. after TAVI and P = 0.069 for baseline vs. peak dobutamine.
Repeated measures ANOVA P < 0.01 for before vs. after TAVI but P = 0.13 for baseline vs. peak dobutamine.
Pre-transcatheter aortic valve implantation physiology
Before TAVI, mean transvalvular pressure loss (ΔP) did not display a consistent relationship with transvalvular flow (Q). Neither linear (median R2 0.16) nor quadratic (median R2 < 0.01) models using resting measurements fit the entire range of data well, implying that a severely stenotic aortic valve does not predictably behave like a pure resistor or orifice. Even a model with both viscous and separation components using all observations, as classically used for vascular stenoses,8,9 fit the population only modestly (median R2 0.43), indicating that haemodynamic pathophysiology of a dynamic valvular stenosis differs fundamentally from a fixed coronary stenosis.
Within our conceptual framework, we found all five expected patterns of ΔP vs. Q before TAVI. Whereas few cases (3, or 20%) behaved like an orifice or worse, a large majority of cases (10, or 67%) fit a linear or sublinear pattern. These results imply that an orifice model for AS physiology6 applies to a small number of cases, and that even severely stenotic aortic valves commonly show favourable dynamic physiologic changes with dobutamine stress toward reduced severity. Take home figure shows the conceptual framework as well as clinical examples of three key patterns.
Take home figure.
Conceptual framework for aortic stenosis physiology. The shape of curve linking systolic ejection transvalvular pressure gradient (ΔP) to transvalvular flow (Q) provides a physiologic ‘fingerprint’ of haemodynamics unique to that stenotic valve. A single rest measurement (coloured blue) cannot predict which path will be observed during dobutamine stress (coloured red). Five patterns of increasing severity can be anticipated, from most severe (worse than the quadratic shape assumed by the Gorlin orifice model) to least severe (better than the linear shape of a resistor). Three examples from the cohort, plus the example in the right panel of Figure 2, provide visual evidence of the heterogeneity of valvular pathophysiology.
Stress aortic valve index
As developed in detail in the Supplementary material online, the stress aortic valve index (SAVI) provides a valve-specific summary of the pressure loss vs. flow curve during maximal physiologic conditions. The stress aortic valve index equals the unitless, mean aortic/left ventricular systolic ejection pressure ratio during peak stress, reflecting the relative pressure loss over the stenotic valve. A value of 1.0 implies no pressure loss, whereas 0.7 indicates that under peak conditions 30% of the driving pressure in the LV is lost across the aortic valve.
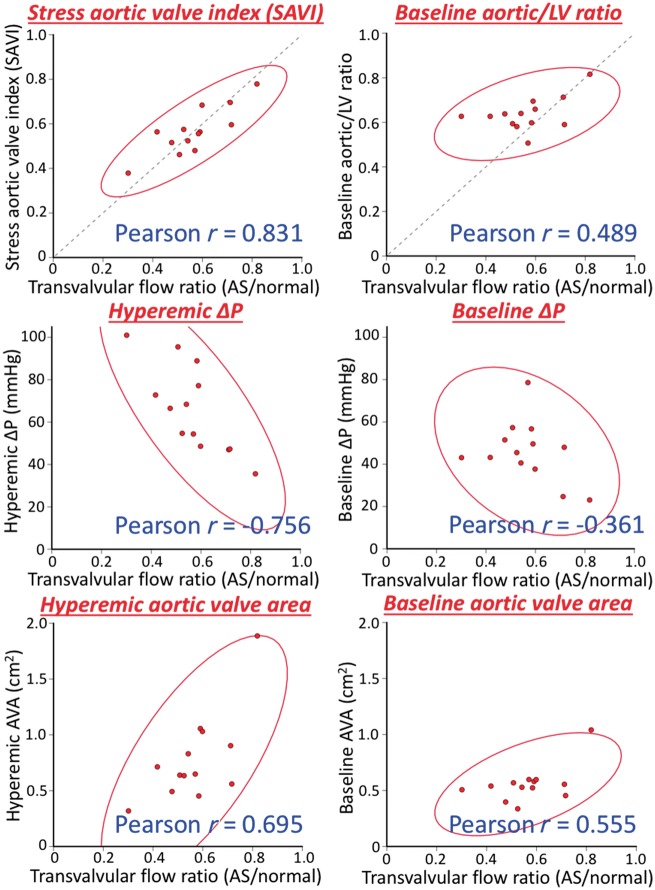
Because minimal systemic vascular resistance during systolic ejection using dobutamine was similar before and after TAVI, median 6.1 [interquartile range (IQR) 4.6–8.7] vs. 4.7 (IQR 4.2–5.8) Woods units (paired Wilcoxon P-value 0.46), SAVI also quantifies the relative reduction in transvalvular flow caused by the stenotic aortic valve, as derived in the Supplementary material online. Figure 3 empirically confirms a progressive hierarchy of correlation between various metrics and the relative reduction in transvalvular flow: SAVI correlates best (Pearson r = 0.83), then hyperaemic ΔP (r = −0.76), hyperaemic AVA (r = 0.70), baseline AVA (r = 0.56), baseline aortic/left ventricular ratio (r = 0.49), and baseline ΔP worst (r = −0.36).
Figure 3.
Relationships with flow reduction from aortic stenosis. For the 13 subjects with successful paired assessments before and after transcatheter aortic valve implantation, we can estimate the flow reduction due to the stenotic aortic valve since systemic vascular resistance during systolic ejection remains constant during peak dobutamine (see Supplementary material online). The stress aortic valve index, equal to the aortic/left ventricular pressure ratio during systolic ejection, shows the best correlation (solid red lines denote 95% confidence ellipses), with stress assessments performing better than resting assessments and unitless ratios performing better than their corresponding absolute gradients with intermediate performance for the aortic valve area.
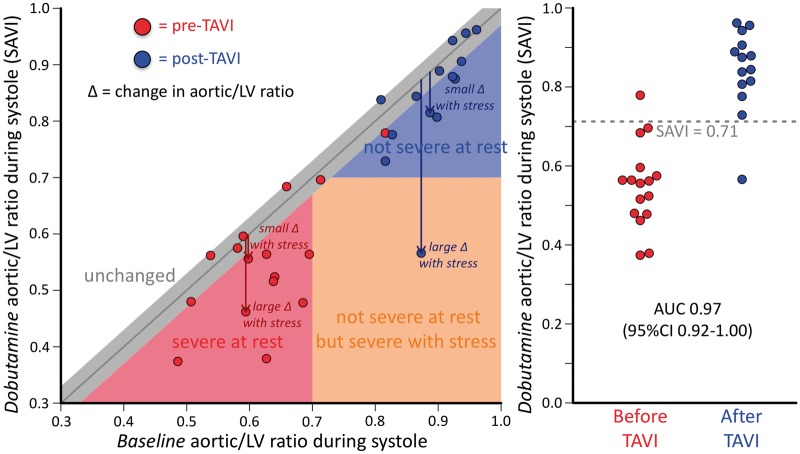
Figure 4 displays the relationship between SAVI (during dobutamine) and the aortic/left ventricular pressure ratio at rest. Many subjects displayed a markedly different SAVI from baseline conditions, demonstrating a heterogeneous response to stress conditions also reflected in the variety of observed patterns for the ΔP vs. Q and dynamic anatomic changes seen in other studies by echocardiography and non-invasive imaging. Baseline clinical factors in Table 1 and resting haemodynamics in Table 2 were not significant predictors of the observed change in the aortic/left ventricular pressure ratio, as detailed in the Supplementary material online. Instead, heterogeneity arises due to a combination of diverse ΔP vs. Q relationships, as in Take home figure, coupled with individualized systemic vascular resistance in response to dobutamine infusion.
Figure 4.
Baseline vs. stress valve haemodynamics. Before transcatheter aortic valve implantation (red points) all subjects except one had an aortic/left ventricular ratio during systole of 0.7 or less. After transcatheter aortic valve implantation (blue points) all implants except one had a stress aortic/left ventricular systolic pressure ratio (stress aortic valve index) greater than 0.7. Heterogeneity existed between baseline and stress aortic valve index measurements; some native valves or implants showed little change (grey area within 0.03 of equality) while others showed large changes. Receiver operating characteristic curve analysis found an optimal threshold at 0.71 to separate pre- and post-transcatheter aortic valve implantation assessments with a large area under the curve of 0.97 (95% confidence interval 0.92–1.0).
Post-transcatheter aortic valve implantation physiology
After TAVI, we observed a highly linear relationship between ΔP and Q. The median R2 of 0.959 implies that almost 96% of the observed variation could be explained by a straight line through the origin. Hence post-TAVI physiology requires only a single parameter, namely the slope of ΔP vs. Q, or valve resistance. Median post-TAVI resistance was 0.65 (IQR 0.41–1.15) Woods units and not significantly different between devices: Edwards 0.41 (IQR 0.36–0.46) vs. Medtronic 0.92 (IQR 0.65–1.22) Woods units, unpaired Wilcoxon P-value 0.059.
Figure 4 compares SAVI values before and after TAVI. Visually a separation exists near 0.7, confirmed by receiver operating characteristic curve analysis that produced an optimal threshold of 0.71 with an area under the curve of 0.97 (95% confidence interval 0.92–1.00). A modest correlation existed between paired SAVI values (Pearson r = 0.59, P = 0.025).
Discussion
This haemodynamic study answers three key questions regarding AS pathophysiology. First, neither orifice nor resistor models alone correctly describe the behaviour of stenotic aortic valves undergoing TAVI. The observed patterns of pressure loss vs. flow curves agree with our systematic framework of flow-dependent stenosis geometry, supported by prior work using echocardiography and tomographic imaging. Second, measurements made under resting conditions in asymptomatic stable patients do not reliably predict haemodynamics during stress conditions when valve-related symptoms may occur. The SAVI, equal to the aortic/left ventricular systolic ejection pressure ratio during dobutamine, offers a quantitative measurement of the relative peak flow limitation through the stenotic valve. By analogy, SAVI provides a ‘fractional flow reserve’ of the aortic valve, unmasking through hyperemia significant stenosis severity not apparent at rest conditions. Third, after TAVI the valve loses the orifice quadratic component through mechanical improvement of the previously stenotic geometry and behaves like a pure linear resistor characterized by a single number—the valve resistance10 or its inverse, valve compliance—that optimally describes post-TAVI physiology.
Invasive,11 echocardiographic,12 and computed tomographic imaging15 literature report changes in AVA with stress. Our application of pressure loss vs. flow curves provides the physiologic associations, mechanisms, and consequences of this dynamic geometry since neither stenotic valves or TAVI devices behave like an orifice. While potentially useful as an anatomic description, AVA does not reflect or summarize the physiologic behaviour of stenotic aortic valves or TAVI devices. Disagreement between anatomic descriptions like AVA and physiologic impact like ΔP or SAVI is not a new observation for either vascular or valvular stenosis. Indeed, much of the current debate regarding ‘low flow, low gradient severe AS’7 reflects this discordance between form and function. As already resolved for coronary stenosis, anatomic metrics like AVA may prove inferior to physiologic metrics like SAVI for AS, although this hypothesis requires testing in future trials.
The observed, unpredictable heterogeneity of pressure gradient vs. flow characteristics in response to stress implies that resting valve haemodynamics cannot reliably substitute for conditions during stress when patients may experience symptoms. Current guidelines restrict a dobutamine ‘valvular stress test’ to limited clinical circumstances, specifically an AVA ≤ 1.0 cm2, resting mean ΔP < 40 mmHg, and ejection fraction < 50%.16 However, the limitations of AVA for predicting significant, stress-induced, abnormal physiology suggest that assessment of the ‘valvular fractional flow reserve’ might reveal a severity potentially suitable for TAVI than is not apparent on resting assessment. Consequently, some portion of patients with exertional symptoms yet only ‘moderate’ stenosis at rest may have a marked increase in pressure loss during dobutamine stress. If this subset of patients achieves a SAVI < 0.7, then Figure 4 implies that their physiologic severity on exertion compares with patients currently undergoing TAVI for indications supported by existing randomized trials.
For quantifying stress valve physiology, SAVI offers several benefits over hyperaemic ΔP. First, as demonstrated in Figure 3, SAVI empirically correlates better than hyperaemic ΔP with the relative reduction in transvalvular flow through the stenotic aortic valve. Second, as derived in the Supplementary material online, SAVI theoretically equals the relative reduction in transvalvular flow over the range of left ventricular driving pressures, whereas hyperaemic ΔP does not account for such variations. Consequently, two patients with identical 30% reductions in transvalvular flow due to AS would have the same SAVI of 0.7 but different hyperaemic ΔP of 36 mmHg (assuming the left ventricular ejection pressure was 120 mmHg) or 45 mmHg (assuming the left ventricular ejection pressure was 150 mmHg). Therefore SAVI accounts for heterogeneity of left ventricular pressure to ensure physiologic comparability among patients, unlike a fixed hyperaemic ΔP threshold of 40 mmHg.
Comparison to existing literature
A study of 20 patients with isolated severe AS (mean baseline gradient 59 ± 4 mmHg) and intact left ventricular performance (mean resting cardiac output 5.4 ± 0.3 L/min, stroke volume index 37 mL/m2) assessed invasive haemodynamics at rest and during supine leg exercise.11 Only five of the 20 (25%) had valve behaviour that matched the quadratic model of Gorlin,6 while the remaining 75% showed a less severe increase than expected during exercise. Our results agree that only a minority of severe AS fits an orifice description. We extended that prior work by defining many more points on the ΔP vs. Q curve, thereby permitting classification into patterns as the basis for: (i) a systematic theoretical framework to explain the results; (ii) the fluid dynamically-based ‘valvular fractional flow reserve’ or SAVI metric of physiologic severity; and (iii) repeating measurements after valve intervention in the same subjects.
A cohort of 50 subjects with asymptomatic, isolated, moderate or severe AS, and normal left ventricular function underwent baseline and dobutamine assessment using echocardiography.17,18 Despite having only two physiologic states for assessment, their results showed ‘considerable variability in individual pressure drop/flow slopes within each group’ of resting severity,17 in agreement with our observed heterogeneity of stress response. Multivariable analysis demonstrated that only stress-related haemodynamics (ΔP, peak velocity, or ΔP/Q slope) significantly predicted progression to symptoms requiring aortic valve replacement during an average 21 months of follow-up,18 consistent with our development of the hyperaemic index SAVI.
In a cohort of 46 subjects with low gradient AS (mean 25 mmHg) but reduced AVA (mean 0.81 cm2), 23 underwent SAVR based on dobutamine echocardiography findings of severe AS.12 Their proposed linear relationship between AVA and transvalvular flow is an equivalent physiologic assumption to a fixed curvilinear shape of the ΔP vs. flow curve, as detailed in our Supplementary material online. When applied to our cohort of high gradient (mean 45 mmHg) severe AS, their fixed model had a reasonable performance before TAVI (median R2 0.70), superior to orifice (median R2 < 0.01), resistor (median R2 0.16), and fixed viscous plus separation (median R2 0.43) descriptions. After TAVI, however, their model had an inferior performance (median R2 0.75) to a linear resistor model (median R2 0.96). Drawbacks to their model of aortic valve physiology include its focus on AVA, shown in Figure 3 to have a weaker correlation with the relative flow reduction caused by the stenotic valve, and its inability to describe the highly linear ΔP vs. flow pattern observed after TAVI.
Limitations
We used dobutamine stress instead of exercise stress due to the patient population and need for general anaesthesia related to the planned TAVI procedure. Given prior literature, we believe our findings extend to exercise11 or dobutamine infusion in awake patients.17,18 While relevant to our population of elderly patients undergoing TAVI, dobutamine may fit even better for younger patients with only moderate AS and primarily exercise-induced symptoms.19 Our cohort was drawn from patients undergoing TAVI for current indications, thereby excluding populations like moderate AS at rest with symptoms, asymptomatic but severe AS, and ‘low flow, low gradient’ severe AS. Therefore, application of dobutamine stress for such patients requires further study.
The sample size of 16, while modest, is consistent with other invasive AS physiology studies in prior decades. For example, the foundational Gorlin study in the early 1950’s did not enroll any AS subjects,6 a prominent paper from the 1970’s had 20 subjects,11 an outcomes study from the mid 1980’s included 16 subjects with AS,20 and a recent manuscript on coronary/valve interactions contained 22 subjects with severe AS.21 Enrolment in our study finished because the final subject suffered a fatal cardiac arrest before TAVI and 2 min after cessation of dobutamine at 20 μg/kg/min. Retrospective analysis demonstrated a baseline aortic/left ventricular pressure ratio of 0.49 and SAVI of 0.37, clearly worse than all other cases. While autopsy did not reveal a cause of death, including no significant coronary atherosclerosis, haemodynamic analysis suggests that continuous and real-time display of aortic/left ventricular pressure ratios during valvular evaluation, and cessation of dobutamine when SAVI reaches 0.50 or coronary perfusion pressure falls, could provide safe yet sufficient physiologic stress.
Finally, we employed invasive haemodynamics with two high fidelity pressure wires to obtain quality data for analysis. In routine practice the substitution of a fluid-filled catheter for the aortic pressure seems reasonable based on our sensitivity analysis in the Supplementary material online, especially if placed in the high aorta to minimize pressure recovery effects.13
Conclusions
Application of pressure loss vs. flow curves demonstrates that neither orifice nor resistor models alone correctly describe AS pathophysiology but rather an individually varying mix of both by our systematic fluid dynamic framework reflecting changing stenosis geometry. Because resting assessment commonly does not reliably predict haemodynamic severity during stress, stress-induced physiologic assessment may offer essential insights into patients with only moderate AS at rest but exertional symptoms for whom resting severity fails to meet current requirements for TAVI.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We gratefully acknowledge the staff of the Catharina Ziekenhuis Eindhoven (CZE) for their dedication, assistance, and patience during the study protocol.
Funding
This work was supported by an institutional grant of commercial pressure wires from St. Jude Medical.
Conflict of interest: N.P.J., D.T.J., R.L.K., and K.L.G. received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis. N.P.J. has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm and has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Volcano/Philips Corporation (DEFINE-FLOW, NCT02328820) for other studies using intracoronary pressure and flow sensors. J.M.Z. reports no support or industry relationships. P.A.L.T. received an institutional grant of commercial pressure wires from St. Jude Medical for this study. P.H. reports no support or industry relationships. F.M.Z. reports no support or industry relationships. R.A.B. serves as a clinical consultant for Philips Research (Eindhoven, Netherlands); received travel funding from CSL Behring to the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA) in 2015; and received fees from AbbVie for preparation of educational material and lectures in 2013. GRGB reports no support or industry relationships. D.T.J. reports no additional support or industry relationships. J.J.K. reports no support or industry relationships. H.H.M.K. serves as a clinical consultant for Philips Research (Eindhoven, Netherlands). I.F.W. reports no support or industry relationships. R.L.K. reports no additional support or industry relationships. N.H.J.P. receives institutional grant support from St. Jude Medical; serves as a consultant for St. Jude Medical, and Opsens; and possesses equity in Philips, GE, ASML, and Heartflow. K.L.G. is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664 and K171303), software packages for cardiac positron emission tomography image processing, analysis, and absolute flow quantification. N.P.J., P.A.L.T., D.T.J., R.L.K., N.H.J.P., and K.L.G. have a patent pending on some of the methods described in this manuscript.
Footnotes
See page 2656 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy224)
References
- 1. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB.. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006–3008. [DOI] [PubMed] [Google Scholar]
- 2. Capodanno D, Leon MB.. Upcoming TAVI trials: rationale, design and impact on clinical practice. EuroIntervention 2016;12:Y51–Y55. [DOI] [PubMed] [Google Scholar]
- 3. Iung B, Laouénan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge P, Fajadet J, Leprince P, Leguerrier A, Lièvre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M; FRANCE 2 Investigators. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart 2014;100:1016–1023. [DOI] [PubMed] [Google Scholar]
- 4. Edwards FH, Cohen DJ, O’brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 5. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators. Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol 2016;68:343–352. [DOI] [PubMed] [Google Scholar]
- 6. Gorlin R, Gorlin SG.. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. Am Heart J 1951;41:1–29. [DOI] [PubMed] [Google Scholar]
- 7. Clavel MA, Magne J, Pibarot P.. Low-gradient aortic stenosis. Eur Heart J 2016;37:2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gould KL, Kelley KO.. Physiological significance of coronary flow velocity and changing stenosis geometry during coronary vasodilation in awake dogs. Circ Res 1982;50:695–704. [DOI] [PubMed] [Google Scholar]
- 9. Young DF, Cholvin NR, Roth AC.. Pressure drop across artificially induced stenoses in the femoral arteries of dogs. Circ Res 1975;36:735–743. [DOI] [PubMed] [Google Scholar]
- 10. Ford LE, Feldman T, Chiu YC, Carroll JD.. Hemodynamic resistance as a measure of functional impairment in aortic valvular stenosis. Circ Res 1990;66:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Bache RJ, Wang Y, Jorgensen CR.. Hemodynamic effects of exercise in isolated valvular aortic stenosis. Circulation 1971;44:1003–1013. [DOI] [PubMed] [Google Scholar]
- 12. Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, Baumgartner H, Beanlands RS, Chayer B, Kadem L, Garcia D, Durand LG, Pibarot P.. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006;113:711–721. [DOI] [PubMed] [Google Scholar]
- 13. Laskey WK, Kussmaul WG.. Pressure recovery in aortic valve stenosis. Circulation 1994;89:116–121. [DOI] [PubMed] [Google Scholar]
- 14. Adele C, Vaitkus PT, Tischler MD.. Evaluation of the significance of a transvalvular catheter on aortic valve gradient in aortic stenosis: a direct hemodynamic and Doppler echocardiographic study. Am J Cardiol 1997;79:513–516. [DOI] [PubMed] [Google Scholar]
- 15. Maragiannis D, Jackson MS, Igo SR, Schutt RC, Connell P, Grande-Allen J, Barker CM, Chang SM, Reardon MJ, Zoghbi WA, Little SH.. Replicating patient-specific severe aortic valve stenosis with functional 3D modeling. Circ Cardiovasc Imaging 2015;8:e003626.. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 17. Takeda S, Rimington H, Chambers J.. The relation between transaortic pressure difference and flow during dobutamine stress echocardiography in patients with aortic stenosis. Heart 1999;82:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeda S, Rimington H, Chambers J.. Prediction of symptom-onset in aortic stenosis: a comparison of pressure drop/flow slope and haemodynamic measures at rest. Int J Cardiol 2001;81:131–139. [DOI] [PubMed] [Google Scholar]
- 19. Cnota JF, Mays WA, Knecht SK, Kopser S, Michelfelder EC, Knilans TK, Claytor RP, Kimball TR.. Cardiovascular physiology during supine cycle ergometry and dobutamine stress. Med Sci Sports Exerc 2003;35:1503–1510. [DOI] [PubMed] [Google Scholar]
- 20. Carabello BA, Williams H, Gash AK, Kent R, Belber D, Maurer A, Siegel J, Blasius K, Spann JF.. Hemodynamic predictors of outcome in patients undergoing valve replacement. Circulation 1986;74:1309–1316. [DOI] [PubMed] [Google Scholar]
- 21. Lumley M, Williams R, Asrress KN, Arri S, Briceno N, Ellis H, Rajani R, Siebes M, Piek JJ, Clapp B, Redwood SR, Marber MS, Chambers JB, Perera D.. Coronary physiology during exercise and vasodilation in the healthy heart and in severe aortic stenosis. J Am Coll Cardiol 2016;68:688–697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.