Figure 3.
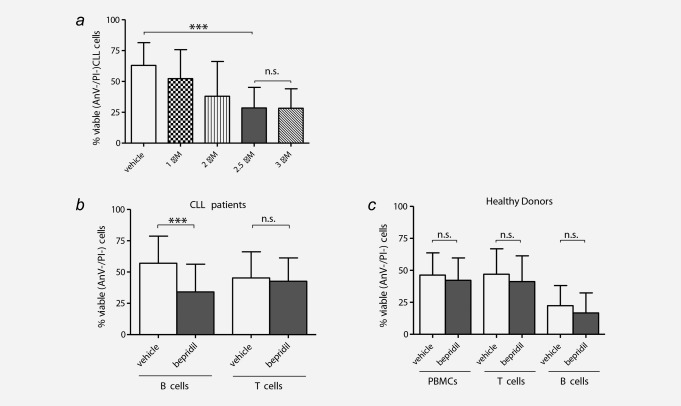
Selective cytotoxic effects of bepridil against primary CLL cells. (a) Primary CLL cells from six cases were incubated in the presence of vehicle control (DMSO) or increasing concentrations of bepridil (1–3 µM) for 24 hr. Viability was determined by Annexin‐V/PI flow cytometry. (b) Primary tumor B (N = 66) and normal T (N = 16) lymphocyte subpopulations from patients with CLL were incubated with 2.5 µM bepridil or vehicle control (DMSO) and viability was assessed at 24 hr culture. Data demonstrated a significant reduction of B CLL viability in bepridil treated compared to vehicle (34%±2.7 vs. 57%±2.6) while T cells were unchanged (42.6% ± 4.6 vs. 45.3% ± 5.2). (c) Peripheral blood mononuclear cells (PBMC) (N = 13), B (N = 10) and T (N = 16) lymphocytes from healthy donors were exposed to bepridil 2.5 µM or vehicle for 24 hr. Viability was evaluated by flow cytometric analysis of Annexin‐V/PI staining. PBMC, B and T cells mean viability values of bepridil treated vs. untreated cells were 41.2% ± 5.5 vs. 46.9% ± 5.5, 16.7 ± 4.9 vs. 22.3 ± 4.9 and 39.1% ± 5 vs. 43.4% ± 4.9, respectively. Results are presented as the percentage of viable (AnV‐/PI‐) cells, mean ± SD, ***p < 0.001 according to Student's test.
