Table 2.
Exploration of the substrate scope of the N‐alkylation of tertiary =NH sulfonimidamide 2 aa: Variation of alkyl halide 9.
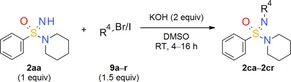
| ||
|---|---|---|
| Alkyl halide (R4Br/I) | Isolated yield [%] | |
| 9 a: bromoethane | 2 ca: 99 | |
| 9 b: 2‐bromopropane | 2 cb: 38 | |
| 9 c: (bromomethyl)cyclobutane | 2 cc: 61 | |
| 9 d: (bromomethyl)cyclopentane | 2 cd: 29 | |
| 9 e: (bromomethyl)cyclohexane | 2 ce: 71 | |
| 9 f: 4‐(bromomethyl)tetrahydro‐2H‐pyran | 2 cf: 60 | |
| 9 g: |
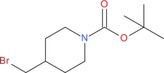
|
2 cg: 35 |
| 9 h: |
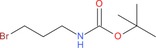
|
2 ch: 50 |
| 9 i: |
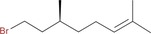
|
2 ci: 46 |
| 9 j: 1‐bromo‐2‐methoxyethane | 2 cj: 80 | |
| 9 k: ethyl bromoacetate | 2 ck: 40 | |
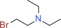
| 9 l: |
|
2 cl: 13 |
| 9 m: methyl iodide | 2 cm: 59 | |
| 9 n: benzyl bromide | 2 cn: 57 | |
| 9 o: |
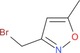
|
2 co: 62 |
| 9 p: allyl bromide | 2 cp: 72 | |
| 9 q: propargyl bromide | 2 cq: 63 | |
| 9 r: 1‐bromobut‐2‐yne | 2 cr: 71 | |
