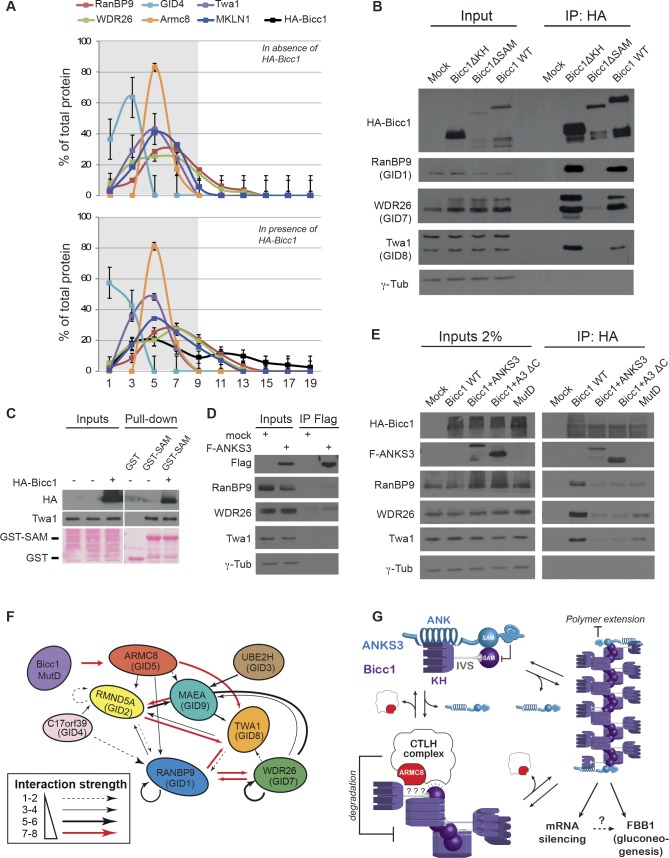
Fig 6. Bicc1 binds the CTLH complex via ARMC8 and dependent on SAM polymer interfaces in competition with ANKS3.
(A) Density fractionation of CTLH complex in extracts of empty vector- (upper graph) or HA-Bicc1-transfected HEK293T cells (below). Graphs show percentages of the total of each subunit per fraction. Grey areas denote LMW fractions 1 to 9. Results represent mean ± SEM from 2 experiments. (B) Anti-HA co-immunoprecipitation of CTLH subunits with HA-Bicc1 or its truncated derivatives Bicc1ΔKH and Bicc1ΔSAM in HEK293T cells. Inputs correspond to 2% of the total extract. Mock-transfected cells were used as negative control. (C) Pull down of endogenous Twa1 by a GST fusion protein of Bicc1 SAM domain or GST alone (control) in HEK293T cells transfected with or without HA-Bicc1. Inputs and IPs on the same gel were cropped because of different exposure times. (D) Anti-Flag co-immunoprecipitation assays in HEK293T cell extracts reveal no CTLH complex binding to Flag-tagged ANKS3 above background levels seen with preimmune IgG. (E) Co-immunoprecipitation of CTLH subunits by HA-Bicc1 alone or together with cotransfected Flag-ANKS3 or C-terminally truncated Flag-ANKS3ΔC, or by Bicc1 MutD. Inputs correspond to 2% of the total extract. Inputs and IPs on the same gel were cropped because of different exposure times. (F) Summary of human CTLH complex subunit interactions and binding to Bicc1 MutD mapped by yeast two-hybrid assays. Arrows depict the relative strength of binding in two independent experiments. (G) Recruitment of CTLH complexes via both ARMC8 and polymerization-competent Bicc1 SAM domains accelerates the clearance of LMW Bicc1 oligomers (left), whereas SAM domain polymerization stabilizes Bicc1 in large scaffolds to mediate silencing of specific target mRNAs and to stimulate FBP1 accumulation (right).