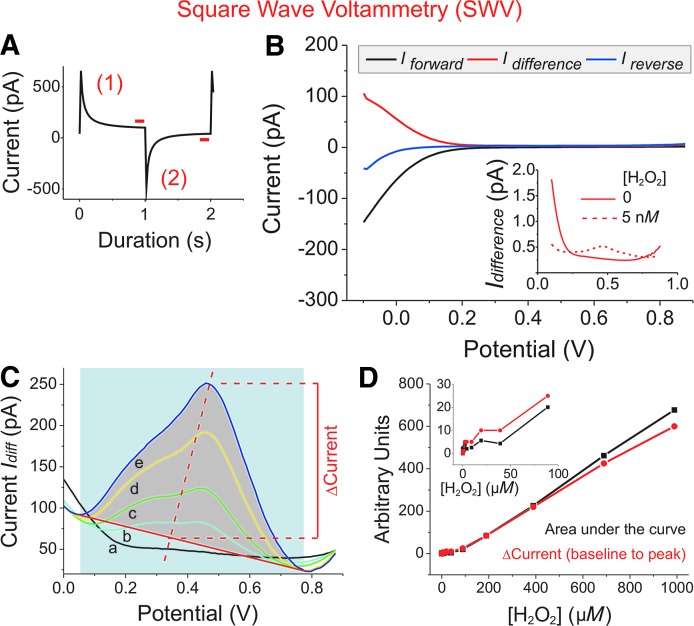
FIG. 2.
H2O2 determination by SWV. Experiments were performed in PBS-I buffer, using a 10 μm Pt-UME versus Ag/AgCl. (A) SWV is a repetitive double-step chronoamperometric technique. For each double-step, two equal, but oppositely directed, potential pulses (±75 mV) were generated, the duration of which was set to τ = 2 s. In a potential window from -0.1 up to +0.9 V mean (nominal) voltage was increased over time by an increment of 10 mV set on top of each positively directed potential step (scan rate = 5 mV/s). Values of the anodic forward (1) and cathodic reverse current (2) were sampled and averaged from the last 10% of the respective plateau phase (red bars). (B) Current traces of a representative SWV experiment in blank buffer. For the net current (Idifference; red traces), Ireverse (measured during the negatively directed potential steps; blue trace) was subtracted from Iforward (measured during the positively directed steps; black trace). The inset with a potential window from 0.1 to 0.9 V shows the characteristic change in the current trace (Idifference) at a mean potential between 470 and 500 mV after H2O2 to a final concentration of 5 nM had been added (dotted line). (C) For calibrating current changes to [H2O2], maxima of Idifference were determined for different [H2O2] (in μM: 990: e, dark blue; 690: d, yellow; 390: c, green; 190: b, light blue; 0: a, black). After definition of a baseline (solid red line), Δcurrent (Ipeak - Ibaseline) was calculated. Alternatively, the size of the area under the curve (gray) was determined. As an example, analysis for the dark blue curve (e) is shown. (D) Δcurrent and area under the curve as determined in (C) were normalized to each other and plotted in arbitrary units against [H2O2]. The inset shows a magnification at lower [H2O2]. SWV, square wave voltammetry.