Abstract
Introduction
Granulomatosis with polyangiitis (GPA) often affects the kidneys, frequently leading to end-stage renal disease (ESRD). Cardiovascular disease (CVD) and infections are common causes of death in GPA and ESRD. Our objective was to examine temporal trends in the mortality of GPA-ESRD in a large nationwide cohort.
Methods
We identified ESRD due to GPA in the US Renal Data System (USRDS) between 1995 and 2014, using nephrologists’ coding for the ESRD etiology. The cohort was divided into four five-year subcohorts based on year of ESRD onset (1995–1999; 2000–2004; 2005–2009; 2010–2014) to assess trends in mortality rates and hazard ratios (HRs) for overall death and cause-specific death, adjusting for potential confounders.
Results
Between 1995 and 2014, there were 5,929 incident cases of GPA-ESRD. The mortality rate (per 100 patient-years) declined from 19.0 in 1995–1999 to 15.3 in 2010–2014 (P=0.01). The adjusted mortality HR of the 2010–2014 cohort was 0.77 (95% CI, 0.66–0.90), compared with the 1995–1999 cohort (P-for-trend <0.001). The corresponding cause-specific mortality HRs after accounting for competing risk were 0.61 (95% CI, 0.47–0.80) for CVD death and 0.42 (95% CI, 0.28–0.63) for infection death (both P-for-trends <0.001).
Conclusion
In this study of nearly all patients who developed ESRD due to GPA in the US over two decades, we found significant improvements in mortality among GPA-ESRD patients. Cause-specific death due to CVD and infections each declined significantly during the study period. These findings are encouraging and likely reflect improved management of both GPA and ESRD.
Granulomatosis with polyangiitis (GPA) is a type of ANCA-associated vasculitis (AAV) that primarily affects small to medium-sized vessels with severe inflammation, leading to major organ damage and premature death. Renal involvement, typically as glomerulonephritis, occurs in up to 70% of patients with GPA and approximately 20–25% of these patients go on to develop end-stage renal disease (ESRD).(1) As with GPA, ESRD is also associated with premature death,(2, 3) especially due to cardiovascular disease (2, 4) and infection;(5, 6) GPA and ESRD are both associated with excess resource utilization.(7, 8) However, the US national trends in the incidence and mortality of ESRD due to GPA (GPA-ESRD) are largely unknown. These data would provide important benchmarks in assessing GPA disease burden and care.
To address these important evidence gaps, we examined temporal trends in the mortality due to GPA due to ESRD in the US from 1995 to 2014 using a national ESRD registry.
Patients and Methods
Data Source and Study Population
The United States Renal Data System (USRDS) is a national registry of ESRD patients, representing an estimated 94% of patients who receive dialysis or kidney transplantation. Patients who refuse replacement therapy, die prior to enrollment, or receive transient dialysis for acute renal failure may not be enrolled. Attending nephrologists are required by law to submit a Medical Evidence Report, which includes the cause of ESRD according to ICD-9 codes, within 45 days of a patient starting a new ESRD treatment. All patients with GPA (ICD-9: 446.4) identified as the cause of ESRD and who initiated ESRD treatment between January 1, 1995 and December 31, 2014 were included in this study. The date of first service for ESRD (ESRD onset) is the earliest of the dialysis start date or date of kidney transplantation. The following information was extracted from the USRDS: demographics (e.g., age, sex, self-reported race); body mass index (BMI) at enrollment; US census region (e.g., northeast, south); relevant comorbidities (e.g., diabetes, hypertension, coronary artery disease); initial ESRD therapy modality; death; and primary cause of death.
US Population Data
To determine GPA-ESRD incidence rates (IRs), annual US population estimates (2000 and 2010 Census estimates and inter-censual population estimates for July of each year for the other years) were obtained from the US Census Bureau. Patients from Puerto Rico, US territories, and foreign countries were excluded from these analyses since US population census estimates do not include these individuals.
Statistical Analysis
Incident GPA-ESRD patients were divided into four five-year subcohorts based on year of ESRD onset (1995–1999; 2000–2004; 2005–2009; 2010–2014) for comparison analyses. For each 5-year period, we calculated annual incidence rates of ESRD due to GPA and its 95% confidence interval (CI). Follow-up for each 5-year subcohort was truncated at the end of each period to avoid the influence of secular trends of the subsequent periods and ensure that subjects across sub-cohorts have the same time interval allowed for follow-up data.(9, 10) We then examined mortality trends during the study period using Poisson regression. In comparing 5-year subcohorts, we used the earlier subcohort (i.e, 1995–1999) as the reference group. Kaplan-Meier survival analysis and log rank test were used to assess differences in the mortality rate over the study period. We employed multivariable Cox regression models to assess mortality trends over the study period while adjusting for potential confounders. We accounted for competing risk of death in our regression models to assess mortality trends of cause-specific death due to cardiovascular disease (CVD), infection, malignancy, and other conditions.(11) All P values were 2-sided with a significance threshold of P less than 0.05. Statistical analyses were performed using SAS, version 9.4.
Sensitivity Analysis
Given the secular trend toward distinguishing MPA (by using ICD-9: 446.0) from GPA in later years, we evaluated whether including patients with ESRD attributed to this ICD-9 code affected temporal trends in ANCA-associated vasculitis (AAV) ESRD incidence and its mortality.
Data Use
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. This study was exempted from the Partner’s HealthCare Institutional Review Board.
Results
Baseline Characteristics
From 1995 to 2014, 5,929 individuals developed ESRD attributable to GPA in the US (Table 1). The overall incidence rate (IR per million US population) of GPA-ESRD increased from 0.81 (95% CI, 0.70–0.92), to 0.94 (95% CI, 0.83–1.05), and to 1.15 (95% CI, 1.03–1.27) for the 1995–1999, 2000–2004, and 2005–2009 subcohorts, respectively (P-for-trend <0.001), but stabilized in the most recent subcohort (1.12, 95% CI, 1.00–1.24, in 2010–2014) compared to the preceding five years (P=0.7). We observed similar trends when GPA and MPA patients were included in the analysis (Supplementary Table 1). The prevalence of diabetes or hypertension among incident GPA-ESRD patients increased (P values <0.05) during the study period, whereas that of coronary artery disease and congestive heart failure declined (P values <0.05). The most frequent initial ESRD therapy throughout the study period was hemodialysis (88 to 92%).
Table 1.
Characteristics of ESRD Patients with Granulomatosis with Polyangiitis in the United States between 1995–2014
| Year of Initial ESRD | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 |
|---|---|---|---|---|
| Number of Cases | 1,104 | 1,345 | 1,728 | 1,752 |
|
|
||||
| Demographics | ||||
|
|
||||
| Age (Mean, SD) | 59.9 (17.2) | 60.1 (18.0) | 61.5 (17.3) | 61.2 (17.1) |
|
|
||||
| Sex (Male %) | 59.3 | 58.1 | 54.5 | 56.3 |
|
|
||||
| Race | ||||
|
|
||||
| White (%) | 88 | 90.6 | 91.2 | 87.9 |
|
|
||||
| Black (%) | 6.0 | 5.4 | 5.9 | 8.2 |
|
|
||||
| Other (%) | 5.9 | 4.0 | 2.9 | 3.9 |
|
|
||||
| BMI (kg/m2, SD) | 25.1 (6.1) | 25.9 (6.2) | 27.2 (6.8) | 27.9 (7.1) |
|
|
||||
| Region* | ||||
|
|
||||
| Northeast (%) | 18.3 | 16.5 | 17.2 | 14.3 |
|
|
||||
| Midwest (%) | 28.6 | 26.8 | 27.7 | 26.1 |
|
|
||||
| South (%) | 35.1 | 36.2 | 34.6 | 38.5 |
|
|
||||
| West (%) | 17.7 | 20.1 | 20.4 | 20.2 |
|
|
||||
| Comorbid Conditions | ||||
|
|
||||
| Diabetes (%) | 7.3 | 7.0 | 19.1 | 20.1 |
|
|
||||
| Hypertension (%) | 52.5 | 61.5 | 74.5 | 76.8 |
|
|
||||
| Coronary Artery Disease (%) | 13.9 | 12.9 | 11.8 | 9.4 |
|
|
||||
| Congestive Heart Failure (%) | 17 | 14.5 | 14.6 | 13.2 |
|
|
||||
| Peripheral Vascular Disease (%) | 4.4 | 4.3 | 5.7 | 5.7 |
|
|
||||
| Stroke or TIA (%) | 3.8 | 4.0 | 4.3 | 5.5 |
|
|
||||
| Current Smoking (%) | 3.5 | 3.8 | 3.5 | 4.5 |
|
|
||||
| History of Malignancy (%) | 4.1 | 4.9 | 5.8 | 5.6 |
|
|
||||
| Initial ESRD Treatment | ||||
|
|
||||
| Transplant (%) | 1.1 | 1.7 | 2.2 | 3.2 |
|
|
||||
| Hemodialysis (%) | 88.3 | 91.7 | 91.6 | 88.8 |
|
|
||||
| Peritoneal Dialysis (%) | 10.6 | 6.6 | 6.2 | 8.0 |
|
|
||||
| Incidence (SD)† | 0.81 (0.70–0.92) | 0.94 (0.83–1.05) | 1.15 (1.03–1.27) | 1.12 (1.00–1.24) |
|
|
||||
TIA=Transient Ischemic Attack;
These four regions capture all of the United States; 24 patents in Puerto Rico; 1 in a US Territory; 5 with Foreign Citizenship;
/1,000,000 US Population;
Mortality Trends
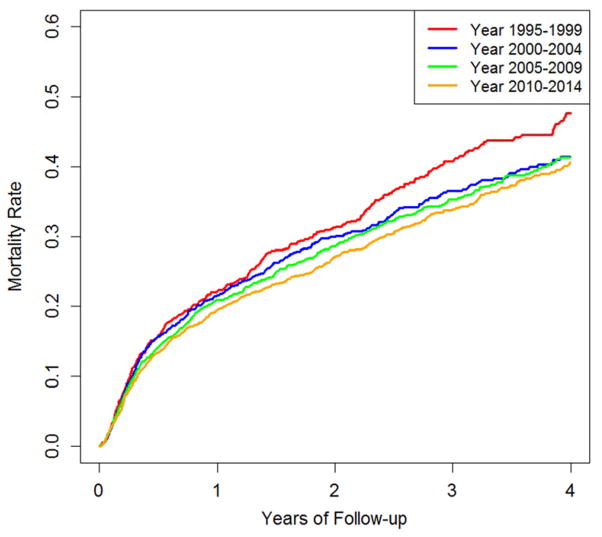
During the study period, 1,795 GPA-ESRD patients died. The mortality rate decreased from 19.0 (95% CI, 17.2–21.1), to 16.9 (95% CI, 15.4–18.7), to 16.2 (95% CI, 14.9–17.7), and to 15.3 (95% CI, 14.0–16.7) per 100 patient-years in the 1995–1999, 2000–2004, 2005–2009, and 2010–2014 subcohorts, respectively (P-for-trend = 0.01) (Table 2). The log-rank test showed significant improvement in survival during the study period (P=0.03) (Figure 1). Age- and sex-adjusted mortality HRs for the 1995–1999, 2000–2004, 2005–2009, and 2010–2014 subcohorts were 1.0, 0.89 (95% CI, 0.76–1.04), 0.80 (95% CI, 0.69–0.93), and 0.75 (95% CI, 0.65–0.87), respectively (P-for-trend <0.001) (Table 2). After adjusting for BMI, smoking, comorbidities, region, and first modality, these HRs did not change materially and remained significant (P-for-trend = 0.005) (Table 2). When patients with GPA and MPA were included in the analysis, the mortality rate showed a similar trend (Supplementary Table 2). Moreover, there was no material change in our results when accounting for whether or not a patient received a transplant during the follow-up period. The age- and sex-adjusted mortality HRs for the 1995–1999, 2000–2004, 2005–2009, and 2010–2014 subcohorts were 1.0, 0.90 (95% CI, 0.77–1.05), 0.82 (95% CI, 0.71–0.95), and 0.76 (95% CI, 0.66–0.88), respectively (P-for-trend <0.001).
Table 2.
Temporal Trends in Risk of Death Among Persons with ESRD due to Granulomatosis with Polyangiitis (1995–2014)
| Year of ESRD Initiation | N | # Deaths* | Incidence of Death/100-patient years (95% CI) | Age- and Sex -Adjusted Hazard Ratio (95% CI) | Multivariate† Adjusted Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| 1995–1999 | 1,104 | 365 | 19.0 (17.2–21.1) | 1.0 (reference) | 1.0 (reference) |
|
|
|||||
| 2000–2004 | 1,345 | 403 | 16.9 (15.4–18.7) | 0.89 (0.76–1.04) | 0.90 (0.77–1.05) |
|
|
|||||
| 2005–2009 | 1,728 | 521 | 16.2 (14.9–17.7) | 0.80 (0.69–0.93) | 0.82 (0.71–0.96) |
|
|
|||||
| 2010–2014 | 1,752 | 506 | 15.3 (14.0–16.7) | 0.75 (0.65–0.87) | 0.77 (0.66–0.90) |
|
|
|||||
| P-for-trend | 0.01 | <0.001 | 0.005 | ||
|
|
|||||
Number of deaths and total follow up determined using the end of the subcohort (e.g., December 31st 1999 for the 1995–1999 subcohort) as the censoring date;
Adjusted for age, sex, BMI, diabetes, hypertension, current smoker, CAD, CHF, CVA, region, and first modality
Figure 1.
Kaplan-Meier Curve of Overall Survival in ESRD due to GPA (1995–2014)
Cause-Specific Death
During the study period, cardiovascular disease (CVD) was the most common cause of death (N=545, 30%) followed by infection (N=286, 16%). Sepsis and pneumonia accounted for 61% and 29% of the infectious causes of death, respectively. After accounting for competing risks (Table 3), the age- and sex-adjusted HRs for death due to CVD in the 1995–1999, 2000–2004, 2005–2009, and 2010–2014 subcohorts were 1.0, 0.84 (95% CI, 0.65–1.08), 0.57 (95% CI, 0.45–0.74), and 0.64 (95% CI, 0.50–0.82, P-for-trend < 0.001), respectively. Similarly, the age- and sex-adjusted HRs for death due to infection in each subcohort were 1.0, 0.84 (95% CI, 0.59–1.19), 0.79 (95% CI, 0.57–1.09), and 0.47 (95% CI, 0.32–0.67, P-for-trend <0.001), respectively. After adjusting for BMI, smoking, comorbidities, region, and first modality, these HRs did not change materially and remained significant.
Table 3.
Cause-Specific Hazard Ratios for Death in ESRD due to Granulomatosis with Polyangiitis (1995–2014)
| Year of ESRD Initiation | N | # Deaths | Unadjusted Hazard Ratio* (95% CI) | Age- and Sex-Adjusted Hazard Ratio (95% CI) | Multivariate Adjusted Hazard Ratio (95% CI)† |
|---|---|---|---|---|---|
|
Cardiovascular Disease
| |||||
| 1995–1999 | 1,104 | 131 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
|
|
|||||
| 2000–2004 | 1,345 | 134 | 0.86 (0.67–1.11) | 0.84 (0.65–1.08) | 0.82 (0.63–1.06) |
|
|
|||||
| 2005–2009 | 1,728 | 131 | 0.61 (0.48–0.79) | 0.57 (0.45–0.74) | 0.54 (0.41–0.71) |
|
|
|||||
| 2010–2014 | 1,752 | 149 | 0.68 (0.53–0.87) | 0.64 (0.50–0.82) | 0.61 (0.47–0.80) |
|
|
|||||
| P-for-trend | <0.001 | <0.001 | <0.001 | ||
|
| |||||
|
Infection
| |||||
| 1995–1999 | 1,104 | 67 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
|
|
|||||
| 2000–2004 | 1,345 | 72 | 0.87 (0.61–1.23) | 0.84 (0.59–1.19) | 0.80 (0.56–1.16) |
|
|
|||||
| 2005–2009 | 1,728 | 93 | 0.85 (0.61–1.18) | 0.79 (0.57–1.09) | 0.74 (0.51–1.06) |
|
|
|||||
| 2010–2014 | 1,752 | 54 | 0.50 (0.35–0.72) | 0.47 (0.32–0.67) | 0.42 (0.28–0.63) |
|
|
|||||
| P-for-trend | 0.001 | <0.001 | <0.001 | ||
|
| |||||
|
Malignancy
| |||||
| 1995–1999 | 1,104 | 9 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
|
|
|||||
| 2000–2004 | 1,345 | 10 | 0.60 (0.26–1.36) | 0.59 (0.26–1.33) | 0.71 (0.28–1.81) |
|
|
|||||
| 2005–2009 | 1,728 | 11 | 0.49 (0.22–1.09) | 0.47 (0.21–1.04) | 0.56 (0.22–1.43) |
|
|
|||||
| 2010–2014 | 1,752 | 15 | 0.65 (0.31–1.36) | 0.63 (0.30–1.33) | 0.71 (0.31–1.63) |
|
|
|||||
| P-for-trend | 0.34 | 0.28 | 0.48 | ||
|
| |||||
|
Other Causes of Death
| |||||
| 1995–1999 | 1,104 | 153 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
|
|
|||||
| 2000–2004 | 1,345 | 187 | 1.10 (0.88–1.38) | 1.07 (0.85–1.34) | 1.08 (0.85–1.38) |
|
|
|||||
| 2005–2009 | 1,728 | 286 | 1.30 (1.05–1.60) | 1.20 (0.97–1.48) | 1.20 (0.96–1.50) |
|
|
|||||
| 2010–2014 | 1,752 | 286 | 1.25 (1.02–1.54) | 1.18 (0.96–1.46) | 1.20 (0.95–1.50) |
|
|
|||||
| P-for-trend | 0.049 | 0.07 | 0.09 | ||
|
|
|||||
Subdistribution hazard function;
Adjusted for age, sex, BMI, diabetes, hypertension, current smoker, CAD, CHF, CVA, region, and first modality
Discussion
In this study of 5,929 patients who developed ESRD due to GPA in the US over the past two decades, we found significant improvements in overall mortality as well as cause-specific death due to CVD and infections, the most common causes in GPA-ESRD. The improved survival of those who developed ESRD due to GPA is encouraging and likely reflects improved management of both GPA and ESRD. In addition, while there were increasing trends in the incidence of new GPA-ESRD cases, rates stabilized in the most recent ten years of the study period. This could reflect advances in the management of patients with GPA in the recent years, as it suggests that fewer GPA patients are progressing to ESRD, given stable GPA incidence itself.(12) Collectively, these findings provide important contemporary benchmarking of the disease burden and outcomes of GPA-ESRD in the US and likely beyond.
The improving motility trends of GPA-ESRD patients agree with a recent study, which found similarly improving trends among 554 patients with AAV and renal disease (before they reached ESRD).(13) The current study findings expand on this observation to describe the improving mortality trends even after the development of ESRD and on a national scale. Furthermore, our study indicates that this overall improvement is largely due to improvement in CVD and infection as causes of deaths, although the previous study did not report these specifics. CVD and infection were reported to be the most common causes of death in GPA-ESRD in smaller cohorts which did not adjust for competing risks.(14–16) Despite these improvements, CVD and infection remain common causes of death in GPA-ESRD. Future studies evaluating cardiovascular risk management and the risks and benefits of immunosuppression in ESRD patients are necessary.
We also found that the incidence of new GPA-ESRD increased until the late 2000’s, but stabilized afterwards. This trend largely agrees with a European study of 2,511 AAV patients from a European dialysis registry (1993–2012). The authors reported that the incidence of AAV-ESRD increased between 1993 and 2007 and slightly decreased afterwards.(16) The initial increasing trend may reflect an initial surge of a large number of survivors with AAV reaching ESRD, while the recent decline may reflect the realization of benefits in improved AAV management over the recent years, including strategies that emphasize maintenance treatment(17) as well as trends in CKD management such as improved blood pressure control. It is also conceivable that earlier recognition and diagnosis of AAV because of the spreading availability of ANCA testing has prevented progression to ESRD among those with renal involvement though studies evaluating temporal trends in delay to diagnosis are unknown to us.
During the study period we also observed significant shifts in the demographics and comorbidities of patients with ESRD due to GPA. The increasing age of ESRD onset in GPA patients observed in our study is consistent with the overall improvements in GPA survival and the risk of indolent progression of CKD to ESRD following the initial insult, perhaps even in the absence of ongoing GPA disease activity.(15) The increasing prevalence of comorbidities such as diabetes, hypertension, and cerebrovascular disease as well as the rising BMI in this cohort is concerning. While these trends likely reflect those in the general population, these findings highlight the importance of assessing and addressing modifiable risk factors in GPA patients to minimize the risk of cardiovascular events in these patients.(17)
This study has several strengths but also limitations. This is a large, population-based study and the first to specifically address temporal trends in survival of GPA-ESRD. Though the USRDS lacks patient identifiers which prohibits one from verifying the diagnosis of GPA, attending nephrologists are required by law to complete medical evidence forms detailing the patient’s medical history and cause of ESRD for the purposes of enrollment in Medicare. As such, the accuracy of the diagnosis is expected to be extremely high, as was the case in lupus ESRD in the same dataset.(18) Furthermore, our observed incidence is consistent with other estimates of GPA-ESRD incidence, supporting the validity of the case definition in our study context.(16, 19) Although the ICD-9 code for MPA is not specific for that condition, we observed similar trends in our primary outcomes regarding incidence and death when we included patients with ESRD attributed to that ICD-9 code in our sensitivity analysis. This suggests that the plateau in incidence in recent years is unlikely to be related to temporal trends in the classification of the cause of ESRD in AAV. Finally, due to a lack of details regarding the diagnosis of GPA, its management, and the treatment of comorbidities (e.g., cardiovascular disease), we are unable to address specific factors responsible for the observed improvement in survival during the study period.
In summary, in this study of nearly all patients who developed ESRD due to GPA in the US over two decades, we found significant improvements in mortality among GPA-ESRD patients. Cause-specific death due to CVD and infections each declined significantly during the study period. These findings are encouraging and likely reflect improved management of both GPA and ESRD.
Supplementary Material
Significance and Innovation.
This is the first study including all patients with ESRD in the US to evaluate mortality among patients with ESRD attributed to GPA;
This study identifies improvements in overall survival as well as CVD- and infection-related death during the study period;
This large population-based study likely captures the exposure and outcomes of interest accurately.
Acknowledgments
Funding:
Dr. Wallace has received funding from a Scientist Development Award from the Rheumatology Research Foundation, an NIH Loan Repayment Award, and the NIH/NIAMS T32-AR007258.
References
- 1.Moiseev S, Novikov P, Jayne D, Mukhin N. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant. 2017 Feb 1;32(2):248–53. doi: 10.1093/ndt/gfw046. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010 Jan;55(1 Suppl 1):S1,420A6–7. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace ZS, Lu N, Unizony S, Stone JH, Choi HK. Improved survival in granulomatosis with polyangiitis: A general population-based study. Semin Arthritis Rheum. 2016 Feb;45(4):483–9. doi: 10.1016/j.semarthrit.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avina-Zubieta JA, Mai A, Amiri N, Dehghan N, Ann Tan J, Sayre EC, et al. Risk of Myocardial Infarction and Stroke in Patients With Granulomatosis With Polyangiitis (Wegener’s): A Population-Based Study. Arthritis Rheumatol. 2016 Nov;68(11):2752–9. doi: 10.1002/art.39762. [DOI] [PubMed] [Google Scholar]
- 5.McGregor JG, Negrete-Lopez R, Poulton CJ, Kidd JM, Katsanos SL, Goetz L, et al. Adverse events and infectious burden, microbes and temporal outline from immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis with native renal function. Nephrol Dial Transplant. 2015 Apr;30(Suppl 1):i171–81. doi: 10.1093/ndt/gfv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Seaghdha CM, Foley RN. Septicemia, access, cardiovascular disease, and death in dialysis patients. Perit Dial Int. 2005 Nov-Dec;25(6):534–40. [PubMed] [Google Scholar]
- 7.Wallace ZS, Lu N, Miloslavsky E, Unizony S, Stone JH, Choi HK. Nationwide Trends in Hospitalizations and In-Hospital Mortality of Granulomatosis with Polyangiitis. Arthritis Care Res (Hoboken) 2016;69(6):915. doi: 10.1002/acr.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klarenbach S, Manns B. Economic evaluation of dialysis therapies. Semin Nephrol. 2009 Sep;29(5):524–32. doi: 10.1016/j.semnephrol.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu N, Peloquin C, Dubreuil M, Neogi T, Avina-Zubieta JA, et al. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis. 2017 Feb;76(2):408–13. doi: 10.1136/annrheumdis-2015-209058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MC, Rai SK, Lu N, Zhang Y, Choi HK. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis. 2017 Jul;76(7):1289–94. doi: 10.1136/annrheumdis-2016-210588. [DOI] [PubMed] [Google Scholar]
- 11.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Watts RA, Al-Taiar A, Scott DG, Macgregor AJ. Prevalence and incidence of Wegener’s granulomatosis in the UK general practice research database. Arthritis Rheum. 2009 Oct 15;61(10):1412–6. doi: 10.1002/art.24544. [DOI] [PubMed] [Google Scholar]
- 13.Rhee RL, Hogan SL, Poulton CJ, McGregor JA, Landis JR, Falk RJ, et al. Trends in Long-Term Outcomes Among Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis With Renal Disease. Arthritis Rheumatol. 2016 Jul;68(7):1711–20. doi: 10.1002/art.39614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidanz F, Day CJ, Hewins P, Savage CO, Harper L. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis. 2007 Jul;50(1):36–46. doi: 10.1053/j.ajkd.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Lionaki S, Hogan SL, Jennette CE, Hu Y, Hamra JB, Jennette JC, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009 Sep;76(6):644–51. doi: 10.1038/ki.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruskova Z, Stel VS, Jayne D, Aasarod K, De Meester J, Ekstrand A, et al. Characteristics and Outcomes of Granulomatosis With Polyangiitis (Wegener) and Microscopic Polyangiitis Requiring Renal Replacement Therapy: Results From the European Renal Association-European Dialysis and Transplant Association Registry. Am J Kidney Dis. 2015 Oct;66(4):613–20. doi: 10.1053/j.ajkd.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016 Sep;75(9):1583–94. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 18.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011 Jun;63(6):1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts RA, Mahr A, Mohammad AJ, Gatenby P, Basu N, Flores-Suarez LF. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant. 2015 Apr;30(Suppl 1):i14–22. doi: 10.1093/ndt/gfv022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.