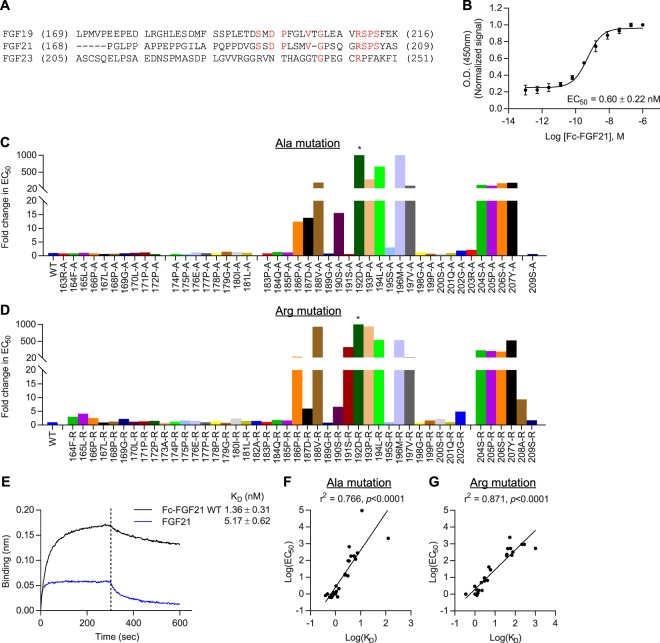
Figure 5.
Two distinct sites in C-terminal region of FGF21 interact with β-Klotho. (A) Amino acid sequence alignment of C-terminal regions of human FGF19, FGF21 and FGF23. Residues that are identical between FGF19, FGF21 and FGF23 are colored red. (B) Binding of WT Fc-FGF21 to soluble human β-Klotho measured by solid-phase binding assay. Results are normalized to the response at the highest concentration of Fc-FGF21 protein. The curve represents mean ± SD of three independent experiments performed in duplicates. (C,D) EC50 values determined from the solid-phase binding assay of Fc-FGF21 (C) alanine and (D) arginine mutants. Values are expressed as fold change relative to WT protein. Gaps were inserted to align the two graphs. *Curve did not converge. (E) Binding of FGF21 and WT Fc-FGF21 to human β-Klotho measured by bio-layer interferometry. KD values were estimated using a 1:1 binding model. (F,G) Linear regression analysis of KD determined by bio-layer interferometry versus EC50 determined by solid-phase binding assay for Fc-FGF21 (F) alanine and (G) arginine mutants. Values represent mean ± SD of three independent experiments.