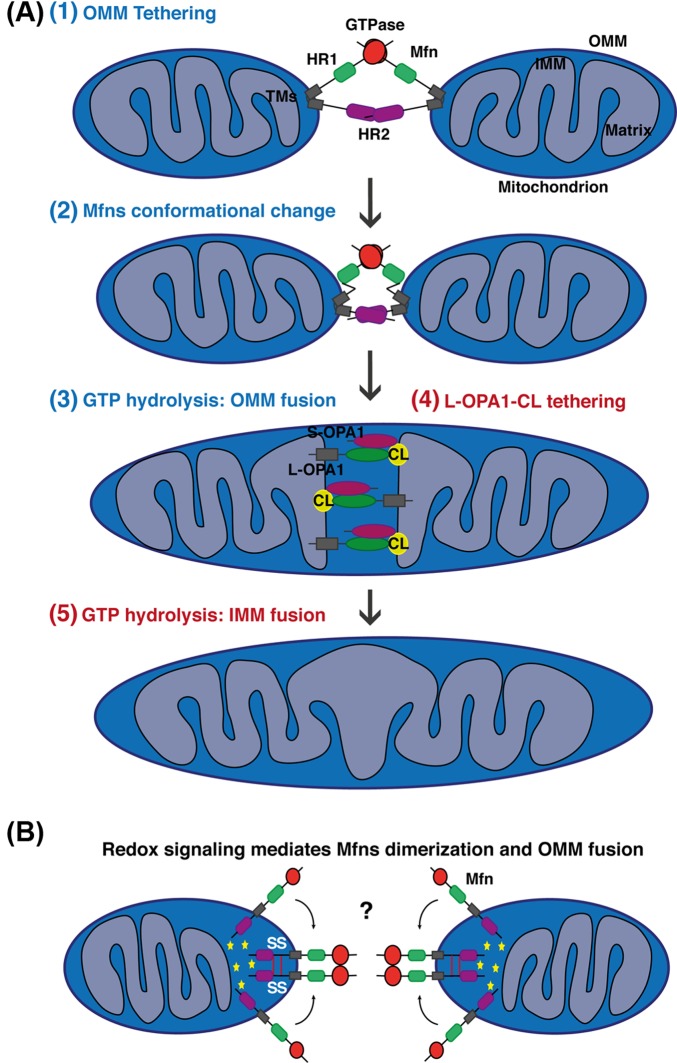
Figure 3. Simplified models for mitochondrial fusion in mammals.
(A) Schematic representations of mitochondrial fusion, based on the Mfns topology suggesting two TM domains with both the HR1 and HR2 domains facing the cytosol. (1) The outer membrane of two opposing mitochondria are tethered by the interaction in trans of the HR2 and/or GTPase domains of Mfns. GTP binding or/and hydrolysis induce Mfns conformational change leading to mitochondrial docking and to an increase of membrane contact sites. For clarity reasons, not all of the recent suggested models leading to Mfns dimerization and conformational change are highlighted in the scheme. (3) Finally, GTPase-dependent power stroke or GTP-dependent oligomerization ensure OMM fusion. The composition of the OMM in phospholipids can also regulate this process. (4) Following OMM fusion, OPA1 and CL drive IMM fusion. The interaction between OPA1 and CL on either side of the membrane tethers the two IMM, which fuse following OPA1-depedent GTP hydrolysis (5). In this model, S-OPA1 has been shown to enhance OPA1–CL interaction and fusion. Please note that after OMM and IMM fusion, Mfn2 and OPA1, as membrane-bound proteins, are still present on the different membranes but are disassembled. (B) Schematic representations of OMM fusion based on the new metazoan Mfns topology suggesting only one TM placing the Mfn C-terminus in the IMS. Oxidized environment in the IMS (ROS production) and increase concentration of GSSG lead to the establishment of two disulphide bonds within the IMS domain. These redox-mediated disulphide modifications induce the dimerization and oligomerization of Mfns molecules which may promote tethering or GTPase activity required for OMM fusion. Interestingly, this redox-regulated Mfns oligomerization is a dynamic and reversible process. Yellow stars indicate an oxidized environment.