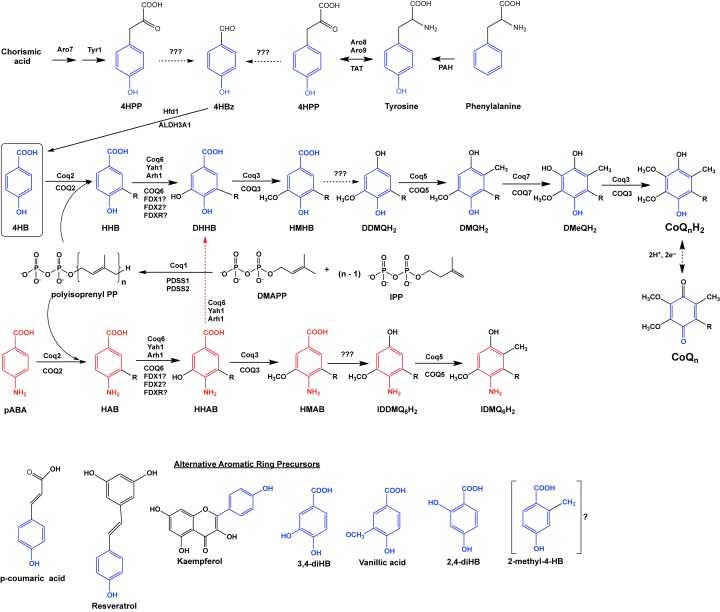
Figure 1. CoQ biosynthetic pathways in the yeast S. cerevisiae and in humans.
The CoQ biosynthetic pathway has been shown to involve at least 14 nuclear-encoded proteins that are necessary for mitochondrial CoQ biosynthesis in S. cerevisiae. Black dotted arrows denote more than one step. Solid arrows denote a single step attributed to the corresponding yeast polypeptide named above each arrow. The corresponding human homologs are named below each arrow. The main ring precursor used by both yeast and humans is 4-hydroxybenzoic acid (4HB). Yeasts synthesize 4HB de novo from chorismate or may obtain it from the metabolism of tyrosine. Humans rely on tyrosine to produce 4HB (or on phenylalanine and phenylalanine hydroxylase to produce tyrosine). Yeast and human cells produce isopentenyl pyrophosphate (IPP) and dimethylally pyrophosphate (DMAPP) as precursors to form hexaprenyl diphosphate (n=6) via Coq1 in yeast or decaprenyl diphosphate (n=10) via PDSS1/PDSS2 in humans. Yeast Coq2 and human COQ2 attach the polyisoprenyl tail to 4HB. Subsequent to this step, the next three intermediates are identified as yeast hexaprenyl-intermediates: HHB, 3-hexaprenyl-4HB; DHHB, 3-hexaprenyl-4,5-dihydroxybenzoic acid; HMHB, 3-hexaprenyl-4-hydroxy-5-methoxybenzoic acid. The next three intermediates are hydroquinones: DDMQH2, 2-hexaprenyl-6-methoxy-1,4-benzenediol; DMQH2, 2-hexaprenyl-3-methyl-6-methoxy-1,4-benzenediol; DMeQH2, 2-hexaprenyl- 3-methyl-6-methoxy-1,4,5-benzenetriol; to ultimately produce the final reduced product (CoQnH2). Red text identifies para-aminobenzoic acid (pABA) as an alternate ring precursor utilized by yeast (but not by humans). The next three intermediates are identified as yeast hexaprenyl-intermediates: HAB, 4-amino-3-hexaprenylbenzoic acid; HHAB, 4-amino-3-hexaprenyl-5-hydroxybenzoic acid; HMAB, 4-amino-3-hexaprenyl-5-methoxybenzoic acid. The next two intermediates are: IDDMQH2, 4-amino-3-hexaprenyl-5-methoxyphenol; IDMQH2, 4-amino-3-hexaprenyl-2-methyl-5-methoxyphenol. The step denoted by the red dotted arrow depends on yeast Coq6 and converts HHAB into DHHB. Interconversion of (CoQnH2) and (CoQn) is shown via a reversible two-electron reduction and oxidation. Steps indicated by ‘???’ are catalyzed by as yet unknown enzymes. Alternative compounds that may serve as ring precursors in CoQ biosynthesis are shown at the bottom of the panel: p-coumaric acid, resveratrol, and kaempferol. Analogs of 4HB that can function to bypass certain deficiencies in the CoQ biosynthetic pathway include: 3,4-dihydroxybenzoic acid (3,4-diHB), vanillic acid and 2,4-dihydroxybenzoic acid (2,4-diHB). It is not yet known whether 2-methyl-4HB (2-methyl-4HB) may also serve a bypass function.