Abstract
Background:
Fatigue is one of the most common symptoms among subjects with chronic obstructive pulmonary disease (COPD), but is rarely identified in clinical practice. The aim of this study was to evaluate the association between fatigue and health-related quality of life (HRQoL) assessed with clinically useful instruments, both among subjects with and without COPD. Further, to investigate the association between fatigue and the COPD Assessment Test (CAT)-energy question.
Methods:
Data were collected in 2014 within the population-based OLIN COPD study. Subjects with (n = 367) and without (n = 428) COPD participated in clinical examinations including spirometry and completed questionnaires about fatigue (FACIT-Fatigue, clinically relevant fatigue ⩽43), and HRQoL (EQ-5D-VAS, lower score = worse health; CAT, lower score = fewer symptoms/better health).
Results:
Subjects with clinically relevant fatigue had worse HRQoL measured with EQ-5D-VAS, regardless of having COPD or not. Decreasing EQ-5D-VAS scores, any respiratory symptoms and anxiety/depression were associated with clinically relevant fatigue also when adjusted for confounders. Among subjects with COPD, clinically relevant fatigue was associated with increasing total CAT score, and CAT score ⩾10. The proportion of subjects with clinically relevant fatigue increased significantly, with a higher score on the CAT-energy question, and nearly 50% of those with a score of 2, and 70% of those with a score of ⩾3, had clinically relevant fatigue.
Conclusions:
Fatigue was associated with respiratory symptoms, anxiety/depression and worse HRQoL when using the clinically useful instruments EQ-5D-VAS and CAT. The CAT-energy question can be used to screen for fatigue in clinical practice, using a cut-off of ⩾2.
Keywords: anxiety, chronic obstructive pulmonary disease, fatigue, health-related quality of life, respiratory symptoms
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health problem with a prevalence estimated at 12% among adults.1,2 Besides respiratory symptoms, fatigue is a common symptom in COPD.3–5 Fatigue increases by disease severity and also in the presence of comorbidities such as heart disease4 and depressive symptoms.6
We have previously shown that fatigue greatly impacts health-related quality of life (HRQoL) among subjects with and without COPD, when assessed with the generic Short Form-36 (SF-36).7 SF-36 is an extensive questionnaire including 36 questions and is thus less suitable for clinical use. There are also shorter generic8 and also disease-specific HRQoL questionnaires.9 These questionnaires have been used for COPD,8–10 but have rarely been evaluated in relation to fatigue.
Besides HRQoL,7 fatigue is also associated with the level of physical activity,11 hospitalization12 and mortality7 among subjects with COPD. Despite the high prevalence and impact of the symptom, fatigue seems to remain unexpressed,13 and is argued to be an important but ignored symptom.14 Fatigue is responsive, for example, to rehabilitation programmes,15 but the lack of simple tools for recognizing fatigue in clinical settings means that possible treatment will not be offered.
There are several instruments to assess fatigue,4,6 but most are too extensive to use in daily clinical practice. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) document recommends the disease-specific COPD Assessment Test (CAT) for assessing the burden of symptoms among patients with COPD.3 According to Jones and colleagues, CAT allows clinicians to identify key areas of health impairments.16 CAT includes eight questions reflecting not only respiratory symptoms and impact on daily life, but also the level of energy. The question regarding level of energy may reflect fatigue, but it has not, as far as we know, been evaluated in relation to validated instruments for assessment of fatigue in a population-based sample.
COPD is an underdiagnosed disease,17,18 but even undiagnosed subjects with COPD have impaired health status,18 and as a consequence, only population-based studies can evaluate the burden of disease. Thus, the primary aim was to evaluate the association between fatigue and HRQoL, assessed with clinically useful instruments, among subjects with and without COPD, and by COPD disease severity, in a population-based study. The secondary aim was to investigate the association between fatigue and the CAT-energy question among subjects with COPD.
Material and methods
Study population
The recruitment of the Obstructive Lung Disease in Northern Sweden (OLIN) COPD study has previously been described in detail.7,11,19 In short, all subjects with obstructive lung function impairment (FEV1/(F)VC < 0.70) were identified from examinations of population-based cohorts in 2002–2004 (n = 993), together with age- and sex-matched referents without obstructive lung function impairment. Since 2005, the study population (n = 1986) has been invited to annual clinical examinations with a basic programme including spirometry and a structured interview.
The present study is based on data collected in 2014. In addition to the basic programme, questionnaires for assessment of fatigue as well as generic and disease-specific HRQoL were distributed (n = 795). For a few subjects, spirometry (n = 21), weight and height (n = 12) were not recorded, and in these cases values from the most recent previous examinations were used (year 2010–2013). Subjects not able to participate in the clinical examination were invited for telephone interview (n = 165). A study flowchart is presented in Figure 1. The study was approved by the Regional Ethical Review Board at Umeå University, Sweden, and the participants provided written informed consent.
Figure 1.
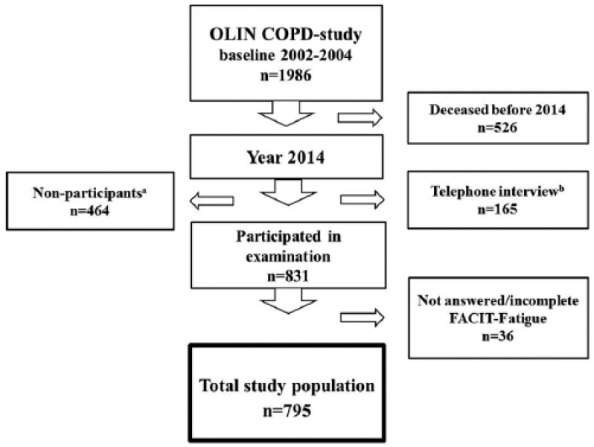
Flowchart for the study population from baseline 2002–2004 to 2014.
aSubjects who refused participation or could not be located at the time of the annual visit.
bSubjects who were not able to attend the clinical examination in 2014.
Spirometric classification
Spirometry was performed according to the ATS guidelines,20 using a dry spirometer, the Dutch Mijnhardt Vicatest 5. COPD was defined according to the GOLD-spirometric criteria,3 FEV1/FVC < 0.70, and disease severity by FEV1 percentage of predicted; GOLD 1: FEV1 ⩾ 80%; GOLD 2: 50% ⩽ FEV1 < 80%; GOLD 3: 30% ⩽ FEV1 < 50%; GOLD 4: FEV1 < 30%. Non-COPD was defined as FEV1/FVC ⩾ 0.70. The highest of pre- or post- bronchodilator values were used, and the OLIN reference values for FEV1.21
Questionnaires
The structured interview questionnaire includes well-validated questions regarding respiratory symptoms,4,11,19 and in addition questions regarding smoking habits and comorbidities. The interview questionnaire also includes the modified Medical Research Council (mMRC)-dyspnoea scale, range 0 to 4, where a higher score indicates more dyspnoea.22 Clinically significant dyspnoea was defined as mMRC score ⩾2.3
FACIT-Fatigue is a 13-item scale estimating degree of fatigue previously used,7,23,24 and validated for COPD.25 The total score ranges from 0 to 52, with higher scores representing less fatigue.26 Clinically relevant fatigue has been defined as a score ⩽43.4
EQ-5D is a validated generic instrument for assessment of HRQoL, applicable to the general population as well as a wide range of health conditions including COPD.8,10 The instrument includes two parts: five questions and a vertical visual analogue scale (VAS) ranging from 0, the worst imaginable health, to 100, the best imaginable health. The VAS scale was used in the current study.
The CAT is a validated disease-specific questionnaire designed to provide a simple measure of health status among patients with COPD.9,16 It consists of eight questions covering cough, phlegm, chest tightness, breathlessness, activity limitations at home, confidence leaving home, sleep and energy. The items are graded from 0 to 5, giving a total score range from 0 to 40, where lower scores indicate fewer symptoms and a better HRQoL.27 A score ⩾10 is defined as a cut-off for burden of symptoms where treatment should be considered.3 In the current study the energy question was also analysed separately and henceforth labelled CAT-energy.
Definitions
Smoking habits were classified as: non-smokers, ex-smokers (stopped at least 12 months ago) and current smokers. Any respiratory symptoms included at least one of the following: chronic cough, chronic productive cough, recurrent wheeze or mMRC dyspnoea ⩾2. Anxiety/depression was defined by affirmative answer to the question ‘Do you have anxiety or depression?’. Heart disease was defined as a self-reported history of a least one of the following: angina pectoris, coronary artery bypass, receiving a percutaneous coronary intervention, myocardial infarction or cardiac insufficiency.
Statistical analyses
SPSS version 24.0 was used for the statistical analyses (IBM, Armonk, NY). Due to a small number of participants, GOLD 3 and 4 were grouped together. Bivariate comparisons between COPD and non-COPD were made with Chi-square test or independent samples t test. Comparisons between COPD and non-COPD across more than two groups were made with the Mantel–Haenszel test-for-trend or analysis of variance (ANOVA). Because of non-normal distribution of FACIT-Fatigue, EQ-5D-VAS and CAT, median scores are presented and the Kruskal–Wallis test was used for comparison of the groups. When significant values were found by the Kruskal–Wallis test, post hoc analyses were made using the Mann–Whitney U test with Bonferroni correction. Spearman’s rho was used to examine the degree of correlation between the questionnaires. A p value of <0.05 was considered statistically significant.
Factors associated with clinically relevant fatigue were analysed stratified for non-COPD and COPD, by logistic regression, and the associations were expressed as odds ratios (OR) with 95% confidence intervals (CI). The dependent variable in all models was clinically relevant fatigue defined as a FACIT-Fatigue score ⩽43. Covariates included were age, sex, FEV1 percentage of predicted, smoking habits, any respiratory symptoms, anxiety/depression, heart disease and health care contacts due to respiratory symptoms. Furthermore, EQ-5D-VAS score (continuous variable), CAT score (continuous variable), CAT score ⩾10, and the CAT-energy question (categories 0–5, with 0 as reference) were included in separate models.
Results
Characteristics of the study population
Subjects with COPD were older, had a lower mean body mass index (BMI), included a higher proportion of current smokers and reported any respiratory symptoms more frequently than subjects without COPD. The proportion of subjects that reported healthcare contacts due to respiratory symptoms were higher in COPD. The prevalence of heart disease and anxiety/depression, respectively, were similar in COPD and non-COPD (Table 1).
Table 1.
Basic characteristics of the study population (n = 795) comparing non-COPD and COPD, and by GOLD stage.
| Characteristic | Non-COPD n = 428 | COPD n = 367 | p value | GOLD 1 n = 177 | GOLD 2 n = 172 | GOLD 3–4 n = 18 | p valuea |
|---|---|---|---|---|---|---|---|
| Female sex, n (%) | 195 (55.7) | 155 (44.3) | 0.346 | 83 (46.9) | 67 (39.0) | 5 (27.8) | 0.051 |
| Age (years), mean (SD) | 70.0 (9.4) | 71.9 (8.9) | 0.004 | 72.1 (9.3) | 71.6 (8.5) | 74.1 (7.8) | 0.502b |
| Age (years), range | 43–94 | 43–94 | 44–94 | 43–94 | 60–89 | ||
| FEV1 (percentage of predicted), mean (SD) | 97.0 (13.8) | 78.7 (16.9) | <0.001 | 92.3 (9.6) | 68.7 (8.4) | 41.0 (7.0) | <0.001 b |
| BMI (kg/m2), mean (SD) | 27.6 (4.3) | 26.8 (4.3) | 0.005 | 26.3 (3.6) | 27.3 (4.7) | 25.7 (4.9) | 0.036 b |
| Current smokers, n (%) | 27 (6.3) | 68 (18.5) | <0.001 | 22 (12.4) | 39 (22.7) | 7 (38.9) | 0.001 |
| mMRC Dyspnea scale ⩾2, n (%) | 6 (1.5) | 44 (13.1) | <0.001 | 13 (16.2) | 25 (16.2) | 6 (37.5) | 0.001 |
| Chronic cough, n (%) | 136 (31.9) | 183 (50.3) | <0.001 | 66 (37.9) | 103 (59.9) | 14 (77.8) | <0.001 |
| Chronic productive cough, n (%) | 134 (31.5) | 188 (51.4) | <0.001 | 77 (43.5) | 96 (56.1) | 15 (83.3) | <0.001 |
| Recurrent wheeze, n (%) | 30 (7.0) | 71 (19.3) | <0.001 | 16 (9.0) | 49 (28.5) | 6 (33.3) | <0.001 |
| Any respiratory symptom, n (%) | 174 (41.6) | 238 (65.7) | <0.001 | 90 (51.7) | 132 (77.6) | 16 (88.9) | <0.001 |
| Anxiety/depression, n (%) | 50 (11.7) | 54 (14.8) | 0.205 | 22 (12.4) | 31 (18.1) | 1 (5.6) | 0.155 |
| Heart disease, n (%) | 61 (14.3) | 67 (18.4) | 0.124 | 25 (14.3) | 39 (22.7) | 3 (16.7) | 0.116 |
| Clinically relevant fatigue, n (%) | 120 (28.0) | 137 (37.3) | 0.005 | 50 (28.2) | 75 (43.6) | 12 (66.7) | <0.001 |
| Healthcare contacts, n (%)c | 35 (8.2) | 64 (17.4) | <0.001 | 18 (10.2) | 39 (22.7) | 7 (38.9) | <0.001 |
Test for trend . bOne-way ANOVA. cDue to respiratory symptoms (last 12 months). Significant values in bold.
ANOVA, analysis of variance; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; mMRC, modified Medical Research Council.
The median FACIT-Fatigue score was lower among subjects with than without COPD (47.0 versus 48.0, p = 0.006), and GOLD 2 and GOLD 3–4 had lower scores than non-COPD (45.0 versus 48.0, p = 0.006 and 38.0 versus 48.0, p = 0.012). The proportion of clinically relevant fatigue was significantly higher among subjects with than without COPD, and increased by GOLD stage (Table 1). The median score for EQ-5D-VAS was similar in non-COPD and COPD, while there was a significant difference when comparing the distributions within the two groups (median 80.0, IQR 71.0–90.0 versus median 80.0, IQR 68.0–90.0, p = 0.002). Median EQ-5D-VAS differed significantly between GOLD stages; GOLD 1: 81.0, GOLD 2: 75.0, and GOLD 3–4: 72.5 (p = 0.004).
FACIT-Fatigue and EQ-5D-VAS among subjects with and without COPD
Moderate to fairly strong correlations were found between the instruments FACIT-Fatigue and EQ-5D-VAS both among subjects with (Spearman’s rho = 0.63, p < 0.001) and without COPD (Spearman’s rho = 0.65, p < 0.001), and within categories of disease severity; Spearman’s rho GOLD 1: 0.51 (p < 0.001), GOLD 2: 0.69 (p < 0.001), GOLD 3–4: 0.54 (p = 0.032). The median EQ-5D-VAS scores were significantly lower among subjects with than without clinically relevant fatigue, both in non-COPD and in each of the GOLD stages (Figure 2).
Figure 2.
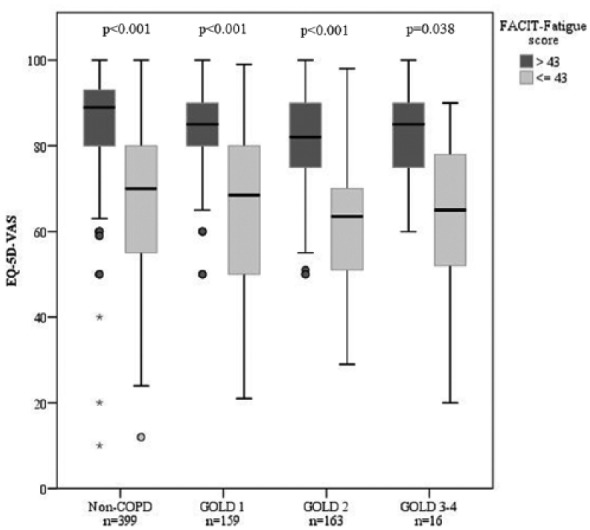
Box plots illustrating median EQ-5D-VAS score (interquartile range) comparing study participants with (FACIT-Fatigue score ⩽43) and without clinically significant fatigue among non-COPD and by COPD disease severity.
CAT and FACIT-Fatigue among subjects with COPD
The median CAT score among subjects with COPD was 9.0. Significant differences were found between the GOLD stages (GOLD 1: 6.0, GOLD 2: 10.0, GOLD 3–4: 17.0, p < 0.001). The proportion of subjects with a CAT score ⩾10 was 42.1%, and increased by disease severity: GOLD 1: 28.7%, GOLD 2: 55.2% and GOLD 3–4: 82.4% (p < 0.001).
Correlations between FACIT-Fatigue and CAT were significant among all subjects with COPD (Spearman’s rho = −0.60, p < 0.001), and in each of the GOLD stages; Spearman’s rho GOLD 1: –0.47 (p < 0.001), GOLD 2: –0.66 (p < 0.001), GOLD 3–4: –0.60 (p = 0.011). The median CAT scores and the proportion of subjects with CAT score ⩾10 were higher among subjects with clinically relevant fatigue than among those without (14.0 versus 6.0, p < 0.001, and 63.5% versus 36.5%, p < 0.001). The findings were similar in each of the GOLD stages (data not shown).
Factors associated with clinically relevant fatigue among subjects with and without COPD
Unadjusted and adjusted analyses of factors associated with clinically relevant fatigue are shown in Tables 2 and 3. Clinically relevant fatigue was associated with any respiratory symptoms, anxiety/depression and decreasing EQ-5D-VAS score, among both subjects with and without COPD, also when adjusted for confounders (Table 2). In similar analyses performed among subjects with COPD only, clinically relevant fatigue remained associated with increasing CAT score, and CAT score ⩾10 independent of age, FEV1 percentage of predicted, anxiety/depression and heart disease (Table 3, Models 1 and 2).
Table 2.
Factors associated with clinically relevant fatigue among subjects with and without COPD, expressed as odds ratios (OR) with 95% confidence intervals (CI); unadjusted and adjusted analyses by logistic regression.
| Unadjusted analyses |
Adjusted analyses |
|||
|---|---|---|---|---|
| Non-COPD |
COPD |
Non-COPD |
COPD |
|
| OR (95% CI) | OR (95% CI) | |||
| Agea | 1.03 (1.01–1.06) | 1.04 (1.02–1.07) | 1.02 (0.99–1.05) | 1.02 (0.98–1.05) |
| Female sex | 1.02 (0.67–1.55) | 0.92 (0.60–1.41) | ||
| FEV1 percentage of predicteda | 0.98 (0.96–0.99) | 0.97 (0.96–0.98) | 1.00 (0.98–1.02) | 0.98 (0.96–1.00) |
| Ex-smoker | 1.07 (0.69–1.65) | 1.20 (0.74–1.95) | ||
| Current smoker | 0.58 (0.21–1.61) | 1.18 (0.63–2.19) | ||
| Any respiratory symptoms | 2.98 (1.91–4.64) | 3.40 (2.05–5.65) | 1.80 (1.04–3.13) | 2.16 (1.02–4.28) |
| Anxiety/depression | 5.84 (3.13–10.91) | 2.40 (1.33–4.30) | 3.06 (1.43–6.55) | 2.39 (1.11–5.13) |
| Heart disease | 1.81 (1.03–3.20) | 2.84 (1.65–4.89) | 0.66 (0.30–1.44) | 1.59 (0.76–3.33) |
| Healthcare contactsb | 2.03 (1.01–4.11) | 1.63 (0.95–2.81) | ||
| EQ-5D-VASa | 0.92 (0.90–0.94) | 0.91 (0.89–0.93) | 0.93 (0.91–0.95) | 0.92 (0.90–0.94) |
| CATa | 1.24 (1.78–1.30) | |||
| CAT ⩾10 | 8.71 (5.34–14.21) | |||
| CAT-energy 1c | 1.74 (0.72–4.19) | |||
| CAT-energy 2c | 6.05 (2.64–13.83) | |||
| CAT-energy 3c | 18.44 (7.03–48.35) | |||
| CAT-energy 4c | 23.60 (6.79–82.01) | |||
| CAT-energy 5c | 36.88 (3.81–357.10) | |||
Entered as a continuous variable. bDue to respiratory symptoms (last 12 months). cCAT-energy 0 as reference. Significant associations in bold.
CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease.
Table 3.
Factors associated with clinically relevant fatigue among subjects with COPD, expressed as odds ratios (OR) with 95% confidence intervals (CI); adjusted analyses in logistic regressions models (Models 1–3 evaluating CAT total score, CAT score ⩾10, the CAT-energy score, respectively).
|
Adjusted analyses
|
|||
|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Agea | 1.04 (1.01–1.07) | 1.04 (1.01–1.07) | 1.05 (1.02–1.08) |
| FEV1 percentage of predicteda | 0.99 (0.98–1.01) | 0.99 (0.97–1.00) | 0.99 (0.97–1.00) |
| Any respiratory symptomsb | 0.81 (0.43–1.53) | 1.46 (0.77–2.75) | 1.87 (1.01–3.44) |
| Anxiety/depression | 2.26 (1.09–4.68) | 2.33 (1.16–4.70) | 2.38 (1.18–4.81) |
| Heart disease | 2.26 (1.16–4.44) | 2.58 (1.34–4.96) | 2.53 (1.31–4.89) |
| CATa | 1.21 (1.15–1.29) | ||
| CAT ⩾10 | 6.54 (3.72–11.50) | ||
| CAT-energy 1c | 1.71 (0.65–4.48) | ||
| CAT-energy 2c | 4.79 (1.90–12.12) | ||
| CAT-energy 3c | 15.32 (5.24–44.80) | ||
| CAT-energy 4c | 17.77 (4.54–69.56) | ||
| CAT-energy 5c | 29.66 (2.56–344.15) | ||
Entered as a continuous variable. bWhen any respiratory symptoms was admitted from Models 1 and 2, the other estimates remained at the same level. cCAT-energy 0 as reference. Significant associations in bold.
CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease.
Clinically relevant fatigue and CAT-energy among subjects with COPD
The proportion of subjects with clinically relevant fatigue increased by increasing CAT-energy score: 0: 11.9%; 1: 19.1%; 2: 45.0%; 3: 71.4%; 4: 76.2%; and 5: 83.3% (p < 0.001). Figure 3 illustrates median (IQR) FACIT-Fatigue score by CAT-energy score, with a line depicting clinically relevant fatigue; CAT-energy scores of ⩾3 were below the cut-off for clinically relevant fatigue. When analysed in a logistic regression model, CAT-energy scores ⩾2 were significantly associated with clinically relevant fatigue independent of age, FEV1 percentage of predicted, any respiratory symptoms, anxiety/depression and heart disease (Table 3, Model 3).
Figure 3.
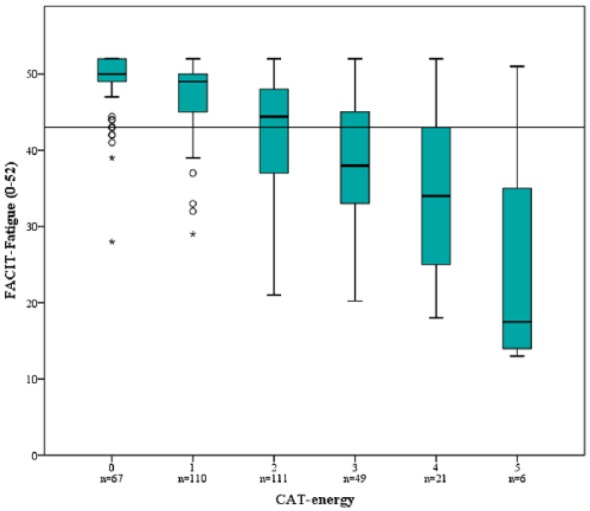
Box plots illustrating median FACIT-Fatigue score (IQR) among subjects with COPD by CAT-energy question score (0–5, higher score indicating less energy). The line depicts clinically relevant fatigue (FACIT-Fatigue score ⩽43).
Kruskal–Wallis test comparing FACIT-Fatigue score between the CAT-energy scores 0–5, p < 0.001.
Discussion
In this population-based study, clinically relevant fatigue was related to worse HRQoL among both subjects with and without COPD, and in all GOLD stages. Clinically relevant fatigue was also independently associated with respiratory symptoms and anxiety/depression. Among subjects with COPD, clinically relevant fatigue was associated with increasing CAT score as well as CAT score ⩾10 independent of age, FEV1 percentage of predicted, anxiety/depression and heart disease. CAT-energy question scores ⩾2 were associated with clinically relevant fatigue independent of confounders. Thus, CAT-energy score seems to be a marker of clinically relevant fatigue, and may be a screening tool for fatigue among patients with COPD in clinical practice.
In line with more complex generic HRQoL questionnaires,7 also the simple EQ-5D-VAS could demonstrate that subjects with clinically relevant fatigue had a worse HRQoL than those without, both among subjects with and without COPD. Similar results were found in a hospital sample of COPD patients when using the disease-specific and comprehensive St. George’s Respiratory Questionnaire; HRQoL was worse among fatigued patients.6 The EQ-5D-VAS may be an easy-to-use tool compared to more time-consuming instruments when evaluating HRQoL among patients with COPD in clinical practice.10 According to our results, it also seems to be sensitive enough to detect impaired health status due to fatigue in a population derived from a population-based setting including mainly mild to moderate COPD.
The disease-specific CAT questionnaire is, in accordance with the GOLD document, recommended to assess the burden of symptoms and HRQoL among patients with COPD in clinical practice.3 The CAT scores can indicate acute deterioration in HRQoL, exacerbations, depressions and mortality among patients with COPD according to a recently published review.9 Furthermore, the CAT scores had a fairly strong correlation with FACIT-Fatigue scores in a primary care population of COPD,27 and explained almost 50% of the variation in FACIT-Fatigue in the current study. To the best of our knowledge, our study is the first to demonstrate that there is an association between clinically relevant fatigue and CAT scores among subjects with COPD in a population-based sample. Both increasing CAT score and a CAT score ⩾10 were associated with clinically relevant fatigue, independent of anxiety/depression, heart disease and other confounders. These results suggest that a higher CAT score – that is a higher burden of symptoms and worse HRQoL – is associated with fatigue.
In our study, FEV1 percentage of predicted was not an independent risk factor for clinically relevant fatigue, while respiratory symptoms and comorbidities seems to be of importance among subjects with and without COPD. The relationship between airflow limitation and HRQoL seems to be weak,3,28 and similar findings have been found among stable patients with COPD – that is, there were no correlations between fatigue and disease severity assessed as FEV1 percentage of predicted or GOLD staging.24 In the latter study, the lack of correlation between fatigue and severity of disease was suggested to be related to an adaptation to chronic impairment. In our COPD population, clinically relevant fatigue was independently associated with anxiety/depression and heart disease. These are common comorbidities in COPD, known to be associated with fatigue,3,4,6,7,24 which highlights the importance of identifying and treating comorbidities that are related to fatigue to relieve the symptom.6 In the current study anxiety/depression was defined using the simple question ‘Do you have anxiety or depression?’, while most others have used more complex anxiety and depression questionnaires.6,24 Thus a positive answer to a simple question regarding anxiety/depression may serve as a gateway for further evaluation of depressive symptoms and fatigue.
Even though fatigue is common in COPD and has an impact on health status, it is often unexpressed13 and not recognized,14 which is why active screening for fatigue is important.5,7 It is known that fatigue can be improved by physical activity, relaxation exercises and other non-pharmacological interventions.15,29,30 By recognizing fatigue in patients with COPD, individual treatment regimens can be initiated and can contribute to improved HRQoL. In the current study the CAT-energy question was associated with fatigue; the proportion of subjects with clinically relevant fatigue increased by increasing CAT-energy score. Almost every other subjects with a score of 2 in the CAT-energy question had clinically relevant fatigue, and more than two out of three with CAT-energy score 3 or higher. Thus the CAT-energy question may be a simple screening tool for fatigue among patients with COPD, and we suggest a cut-off point of 2–3 to be further evaluated.
Strengths and limitations
The strengths of this study are the use of both generic and disease-specific well-validated short and simple instruments useful in clinical practice.10,16 Furthermore, the spirometric criteria for COPD were based on post-bronchodilator spirometry as recommended by guidelines.3 Another strength is the population-based study design; our COPD population is dominated by mild to moderate disease, corresponding to the distribution of severity in the general population.3 The results are not affected by the well-known underdiagnosis17,18 and are thus expected to be generalizable to COPD in society.
There are also limitations. Data were obtained from a longitudinal study in which 526 subjects died before 2014 and a healthy survivor effect must be taken into account. In a previous publication based on the same cohort it was reported that the deceased subjects were older, and had a higher prevalence of productive cough and heart disease, which strengthens the assumption of a healthy survivor effect.31 However, the consequence is that the results may be underestimated, and despite an expected healthy survivor effect there were relationships strong enough to show statistical significance.
Another limitation is that we used reported health care contacts due to respiratory symptoms as a proxy for exacerbations, and there was no obvious association with fatigue. This finding contrasts with others, as increased fatigue has been related to exacerbation frequency,24 and is a strong risk factor for hospitalization and length of hospital admission.12 In other words, fatigue has been shown to be associated to outcomes that are important for the economic burden in COPD.32 However, there is a lack of studies about fatigue in relation to healthcare costs, a context that requires further studies.
Conclusion
In this population-based study, clinically relevant fatigue was associated with worse HRQoL among subjects with and without COPD when using the clinically useful generic instrument EQ-5D-VAS. Respiratory symptoms and anxiety/depression seem to have important impacts, since these factors were independently associated with clinically relevant fatigue. Among subjects with COPD, clinically relevant fatigue was independently associated with increasing CAT score, CAT score ⩾10, increasing age, anxiety/depression and heart disease. However, disease severity, expressed as FEV1 percentage of predicted, did not remain associated with clinically relevant fatigue when adjusted for confounders. The CAT-question evaluating patients’ energy levels was associated with clinically relevant fatigue; about every other with a score of 2, and more than two out of three with a score ⩾3 were affected. We suggest that the CAT-energy question may be a possible screening tool for fatigue among patients with COPD, using a cut-off of 2–3 points.
Acknowledgments
Acknowledgement is given to the participants in the OLIN studies, and to the former and current head of the OLIN studies, Professor Bo Lundbäck and Professor Eva Rönmark. Acknowledgement is also given to the research nurses Ann-Christine Jonsson, Sigrid Sundberg and Britt-Marie Eklund for collecting data, as well as Ola Bernhoff for administering the database.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was supported by grants from the Swedish Heart-Lung Foundation Grant number 20130277, a regional agreement between Umeå University and Västerbotten County Council (ALF) Grant number ALFVLL-225011, VISARE NORR Fund: Northern County Councils Regional Federation Grant number 22/2010, the Swedish Heart and Lung Association, and the County Council of Norrbotten, Sweden.
ORCID iD: Caroline Stridsman  https://orcid.org/0000-0001-6622-3838
https://orcid.org/0000-0001-6622-3838
Contributor Information
Caroline Stridsman, Department of Health Sciences, Division of Nursing, Luleå University of Technology, The OLIN Studies, Robertsviksgatan 9, Luleå, S-971 89, Sweden.
My Svensson, Department of Public Health and Clinical Medicine, The OLIN Unit/Division of Medicine, Umeå University, Umeå, Sweden.
Viktor Johansson Strandkvist, Department of Health Sciences, Division of Health and Rehabilitation, Luleå University of Technology, Luleå, Sweden.
Linnea Hedman, Department of Health Sciences, Division of Nursing, Luleå University of Technology, Luleå, Sweden Department of Public Health and Clinical Medicine, The OLIN Unit/Division of Occupational and Environmental Medicine, Umeå University, Umeå, Sweden.
Helena Backman, Department of Public Health and Clinical Medicine, The OLIN Unit/Division of Occupational and Environmental Medicine, Umeå University, Umeå, Sweden.
Anne Lindberg, Department of Public Health and Clinical Medicine, The OLIN Unit/Division of Medicine, Umeå University, Umeå, Sweden.
References
- 1. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5: 020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backman H, Eriksson B, Rönmark E, et al. Decreased prevalence of moderate to severe COPD over 15 years in northern Sweden. Respir Med 2016; 114: 103–110. [DOI] [PubMed] [Google Scholar]
- 3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD 2017, http://goldcopd.org (2017, accessed October 2017).
- 4. Stridsman C, Muellerova H, Skär L, et al. Fatigue in COPD and the impact of respiratory symptoms and heart disease: a population-based study. COPD 2013; 10: 125–132. [DOI] [PubMed] [Google Scholar]
- 5. Christensen VL, Holm AM, Cooper B, et al. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage 2016; 51: 849–859. [DOI] [PubMed] [Google Scholar]
- 6. Kentson M, Todt K, Skargren E, et al. Factors associated with experience of fatigue, and functional limitations due to fatigue in patients with stable COPD. Ther Adv Respir Dis 2016; 10: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stridsman C, Skär L, Hedman L, et al. Fatigue affects health status and predicts mortality among subjects with COPD: report from the population-based OLIN COPD study. COPD 2015; 12: 199–206. [DOI] [PubMed] [Google Scholar]
- 8. Pickard AS, Wilke C, Jung E, et al. Use of a preference-based measure of health (EQ-5D) in COPD and asthma. Respir Med 2008; 102: 519–536. [DOI] [PubMed] [Google Scholar]
- 9. Karloh M, Fleig Mayer A, Maurici R, et al. The COPD assessment test: what do we know so far? A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest 2016; 149: 413–425. [DOI] [PubMed] [Google Scholar]
- 10. Zanini A, Aiello M, Adamo D, et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care 2015; 60: 88–95. [DOI] [PubMed] [Google Scholar]
- 11. Andersson M, Stridsman C, Rönmark E, et al. Physical activity and fatigue in chronic obstructive pulmonary disease: a population based study. Respir Med 2015; 109: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 12. Paddison JS, Effing TW, Quinn S, et al. Fatigue in COPD: association with functional status and hospitalisations. Eur Respir J 2013; 41: 565–570. [DOI] [PubMed] [Google Scholar]
- 13. Stridsman C, Lindberg A, Skär L. Fatigue in chronic obstructive pulmonary disease: a qualitative study of people’s experiences. Scand J Caring Sci 2014; 28; 130–138. [DOI] [PubMed] [Google Scholar]
- 14. Spruit M, Vercoulen J, Sprangers M, et al. Fatigue in COPD: an important yet ignored symptom. Lancet Respir Med 2017; 5: 542–544. [DOI] [PubMed] [Google Scholar]
- 15. McCarthy B, Casey D, Devance D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J 2009; 34: 648–654. [DOI] [PubMed] [Google Scholar]
- 17. Lindberg A, Bjerg A, Rönmark E, et al. Prevalence and underdiagnosis of COPD by disease severity and attributable fraction of smoking. Report from the Obstructive Lung Disease in Northern Sweden studies. Respir Med 2006; 100: 264–272. [DOI] [PubMed] [Google Scholar]
- 18. Miravitlles M, Soriano JB, Garcia-Rio F. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64: 863–868. [DOI] [PubMed] [Google Scholar]
- 19. Lindberg A, Lundbäck B. The Obstructive Lung Disease in Northern Sweden chronic obstructive pulmonary disease study: design, the first year, participation and mortality. Clin Respir J 2008; 2: 64–71. [DOI] [PubMed] [Google Scholar]
- 20. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995; 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 21. Backman H, Lindberg A, Oden A, et al. Reference values for spirometry: report from the Obstructive Lung Disease in Northern Sweden studies. Eur Clin Respir J 2015; 2: 26375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. [DOI] [PubMed] [Google Scholar]
- 23. Al-shair K, Kolsum U, Dockry R, et al. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir Res 2011; 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baghai-Ravary R, Quint J, Goldring J, et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med 2009; 103: 216–223. [DOI] [PubMed] [Google Scholar]
- 25. Al-shair K, Muellerova H, Yorke J, et al. Examining fatigue in COPD: development, validity and reliability of a modified version of FACIT-F scale. Health Qual Life Outcomes 2012; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cella D. Manual of the functional assessment of chronic illness therapy (FACIT) measurement system. Evanston, IL: Center on Outcomes, Research and Education (Core), Evanston Northwestern Healthcare and Northwestern University, 1997. [Google Scholar]
- 27. Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38: 29–35. [DOI] [PubMed] [Google Scholar]
- 28. Han MK, Muellerova H, Curran-Everett D, et al. Implications of the GOLD 2011 disease severity classification in the COPDGene cohort. Lancet Respir Med 2013; 1: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akgun Sahin Z, Dayapoglu N. Effect of progressive relaxation exercises on fatigue and sleep quality in patients with chronic Obstructive Lung Disease (COPD). Complement Ther Clin Pract 2015; 21: 277–281. [DOI] [PubMed] [Google Scholar]
- 30. Deng GJ, Liu FR, Zhong QL, et al. The effect of non-pharmacological staged interventions on fatigue and dyspnoea in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Int J Nurs Pract 2013; 19: 636–643. [DOI] [PubMed] [Google Scholar]
- 31. Qvist L, Nilsson U, Johansson V, et al. Central arterial stiffness is increased among subjects with severe and very severe COPD: report from a population-based cohort study. Eur Clin Respir J 2015; 2: 103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srivastava K, Thakur D, Sharma S, et al. Systematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary disease. Pharmacoeconomics 2015; 33: 467–488. [DOI] [PubMed] [Google Scholar]


