Abstract
Air pollution is among the top threats to human health in China. As air toxicants, polycyclic aromatic hydrocarbons (PAHs) could bring significant risks to population; however, the exposure to PAHs in China and its health impact are not fully understood. In 2012, a summer exchange program allowed 10 students to travel from Los Angeles to Beijing and stay there for 10 weeks. Based on the program, this study investigated the difference in urinary concentration of 12 hydroxylated-PAHs (Σ12OH-PAHs) and malondialdehyde (MDA) between the two cities. The median concentration of Σ12OH-PAHs in Beijing (14.1 μg g−1 creatinine) was significantly higher than that in Los Angeles (5.78 μg g−1 creatinine), indicating a higher exposure in Beijing. The ratios of homogeneous OH-PAHs (e.g., 1-/2-OH-NAP) changed significantly between the two cities (p < 0.01), which might suggest a potential alteration in metabolism subsequent to exposure. A significant association between Σ12OH-PAHs and MDA (p < 0.01) was observed, with the association varying between the two cities. This study suggests that exposure to PAHs might be linked to metabolism alteration and calls for future studies to investigate the role this possible alteration played in the health effects of PAHs exposure.
Graphical Abstract
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a group of air pollutants that contain two or more fused aromatic rings. Their ubiquitous occurrence in the environment has raised increasing concerns due to their high emissions and significant toxicity. The global emission of PAHs was approximately 504 Gg in 2007, of which 21% was from China.1 PAHs are mainly emitted from combustion sources, such as vehicle emissions, household fuel consumption, and tobacco smoke.2 All of these sources are geographically close to densely populated areas and could therefore bring significant exposure and health risks. Humans are exposed to PAHs through various pathways including inhalation, ingestion, and dermal absorption.2–4 For the assessment of the total exposure to PAHs from different routes, urinary hydroxylated PAHs (OH-PAHs), the metabolites of PAHs, are widely measured.2
PAHs are associated with various adverse health effects (e.g., micronuclei frequency, DNA damage, lung function, and heart rate variability),5–8 and certain adverse health outcomes (e.g., lung cancer, cardiovascular diseases, birth defects, and diabetes).9–12 The biological mechanism of these associations is not yet clear, and oxidative damage is suggested as a possible cause.3,13 It has been shown that reactive oxygen species (ROS) could be generated during the metabolism of PAHs. Then, ROS could attack biological molecules such as DNA, proteins, and lipids, resulting in a series of health problems.6 Malondialdehyde (MDA) is a product of lipid oxidative damage and was widely used as a biomarker for lipid peroxidation.14,15 MDA was previously found to be associated with both PAHs exposure and various diseases,14–16 suggesting a potential role of lipid peroxidation between PAHs and the health effects.
In recent years, the severe air pollution in Beijing has created great concerns.17 As toxic air pollutants, PAHs were also present in higher concentrations in Beijing than in other cities in the developed countries.18–20 In 2012, the University of California, Los Angeles (UCLA) and Peking University (PKU) carried out a summer exchange program in which a panel of 10 UCLA students traveled to Beijing and stayed for 10 weeks, providing an opportunity to study their PAH exposures and related lipid peroxidation. As shown in a previous study, repeated measurements on travelers could allow researchers to focus on the impacts of exposure with less interference from individual differences.21 In this study, the first-morning urine samples of these students were collected before, during, and after the exchange program. A total of 12 urinary OH-PAHs and malondialdehyde (MDA) were measured as surrogates for exposure and lipid peroxidation, respectively. The aims of this study were as follows: (1) assess the exposure to PAHs in Beijing and Los Angeles; (2) characterize the differences in the ratios of OH-PAHs in the two cities to better understand their metabolism; and (3) investigate the association between OH-PAHs and MDA.
MATERIALS AND METHODS
Sample Collection
All 10 subjects (four males and six females) in this study were healthy UCLA students. The age and body mass index (BMI) of the subjects at the time of sample collection were 23.3 ± 5.8 (mean ± standard deviation; range: 20–39, same as below) years and 21.1 ± 1.4 (18.6–23.4) kg m−2, respectively. All the subjects were self-reported nonsmokers and participated in the summer exchange program between UCLA (in Los Angeles) and PKU (in Beijing) in 2012. A total of 11 urine samples were collected for each subject before, during, and after the exchange program, with the specific dates shown in Figure 1. Briefly, three urine samples were collected before the program (LA1, from June 7 to June 19) in Los Angeles. A total of five collections were conducted during the program (BJ, from June 29 to August 8) in Beijing. The last three samples were collected after the program (LA2, from October 8 to October 26) when the students returned to the Los Angeles. Because PAHs are metabolized rapidly in animals and human, with half-lives of less than 1 day,22–24 the urine collection began at least 1 week after arrival in the new city to exclude the interference of previous PAH exposures in the former city. For each urine collection, the first morning urine after fasting for at least 8 h was collected in polypropylene tubes and frozen at −20 °C until analysis.
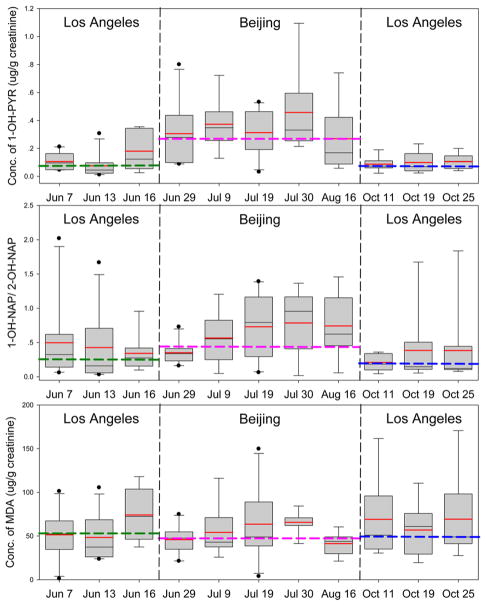
Figure 1.
Temporal trend of 1-OH-PYR, 1-/2-OH-NAP, and MDA in urines. Black line, median of each date; red line, mean of each date; green dashed line, median of Los Angeles before the travel (LA1); pink dashed line, median of Beijing; blue dashed line, median of Los Angeles after the travel (LA2); box, 25th and 75th percentiles; whiskers, 10th and 90th percentiles).
For each subject, a questionnaire was used to collect additional information for the 3 days prior to the sample collection. In the questionnaire, detailed information on cooking behaviors (cooking frequency, cooking fuel, and exposure to barbecuing), diet (the consumption of barbecue or baked meat), traffic-related activities (driving hours, public transportation usage, and duration of stay near heavy traffic areas), and secondhand smoke exposure were collected. This study was performed in accordance with the guidelines and approval of the Institutional Review Boards of both UCLA and PKU, and informed consent was obtained from each subject.
Analytical Method
A previously established method was used in this study to measure the urinary OH-PAHs.14 Briefly, 2 mL of urine from each sample was spiked with 13C-labeled 3-hydroxyphenanthrene (13C-3-OH-PHE) as surrogate standards and adjusted to pH 5.5 with sodium acetate buffer. Next, the sample was added to 20 μL of β-glucuronidase–sulfatase (Helix pomatia, Sigma-Aldrich, St. Louis, MO) and incubated at 37 °C overnight to hydrolyze conjugated phenols. The liquid–liquid extraction with hexane methyl tert-butyl ether mixture (9:1, v/v) was performed three times to extract the analytes. After blowing with nitrogen to near-complete dryness, 0.1 mL of methanol and 1 mL of diazomethane solution were added to the extract, and the OH-PAHs were methylated at room temperature for 5 h. Next, the sample was cleaned with silica gel chromatography (0.6 cm i.d., 6 cm length, with 0.5 cm of anhydrous Na2SO4 on top) and eluted with 8 mL of hexane, 8 mL of hexane dichloromethane mixture (3:2, v/v), and 8 mL of dichloromethane sequentially. The analytes were in the second and third fractions. Finally, the sample was concentrated, spiked with d10-acenaphthene (d10-ACE) and d10-phenanthrene (d10-PHE) as internal standards and analyzed using a gas chromatograph and mass spectrometer (GC-MS; Agilent 7890A-5975C) with an electron ionization (EI) ion source and a 30 m DB-5MS column (250 μm i.d., 0.25 μm film thickness; J & W Scientific, Folsom, CA). The monitored ion couples for all analytes and the method detection limits (MDL) (ranged from 7.5 to 18.2 pg mL−1) are listed in Table S1.
Urinary MDA concentrations were measured based on the reaction with 2-thiobarbituric acid (TBA). Briefly, a 150 μL urine sample mixed with 450 μL of TBA and 900 μL of phosphate (0.5 mol L−1) was incubated in water at 95 °C for 1 h. After being cooled and filtered, the mixture was injected into a high-performance liquid chromatograph (HPLC; Waters 2695) with a reverse-phase C18 column (150 mm in length, 3.9 mm i.d.) and a mobile phase of potassium phosphate (0.05 mol L−1, pH = 6.5) and methanol (60:40, v/v). The MDA-TBA adducts could be detected under a wavelength of 532 nm in a UV detector. The detect limit of the method is 7.2 ng mL−1. Urinary creatinine was measured by a spectrometer under a wavelength of 510 nm based on the Jaffe reaction.
Quality Control
For each batch of eight urine samples, one laboratory blank sample (with 2 mL of purified water) was prepared. The analysis for blank samples was the same as that for urine samples. For all urine samples, three identical samples were prepared to ensure repeatability. The concentrations of 3-hydroxybiphenyl (3-OH-BP, 15.8%), 2,2′-dihydroxybiphenyl (2,2′-DOH-BP, 12.2%), 3,4′-DOH-BP(14.5%), and 3-hydrox-yphenanthrene (3-OH-PHE, 33.9%) in the blank samples were more than 10.0% of the average concentrations in the urine samples and hence removed from the subsequent discussion. The concentrations of the remaining 12 analytes in blank samples were 1.11 ± 1.05% (average ± standard deviation) of the average concentrations in urine samples. Thus, blank subtraction was not performed for all those analytes. The relative deviation of all analytes was 21.0 ± 7.2%. The recovery of 13C-3-OH-PHE was 93.6 ± 12.4%. All the OH-PAHs and MDA data were normalized by creatinine.
Statistical Analysis
The Shapiro–Wilk test was applied to check the normality of the data in this study. Median values (with interquartile range, IQR) were reported for urinary biomarkers and their ratios unless otherwise noted. For analytes not detected in urine samples, the 1/2 MDL was applied as a substitute for the statistical analysis. The Mann–Whitney U-test was used to investigate the difference in urinary biomarkers and questionnaire data between the two cities. A two-tailed p value of <0.05 was considered significant. Multivariate linear regressions with stepwise or enter approaches were applied to identify the confounding factors and calculate the concentration ratios between the two cities. A simple linear regression model and three linear mixed-effects models were used to investigate the association between MDA and OH-PAHs. In the simple linear regression model (Model A), the association between OH-PAHs and MDA was considered constant among subjects in the two cities:
| (1) |
where yijk and xijk are the log-transformed concentrations of MDA and OH-PAHs of subject i at time j in the city k; respectively. α and β is the fixed intercept and slope, respectively. εijk is the residual.
In the three mixed-effects model, a random intercept was allowed among subjects (Model B, eq 2), cities (Model C, eq 3), and both subjects and cities (Model D, eq 4), respectively.
| (2) |
| (3) |
| (4) |
where μi and μk is the random intercept for subject i and city k, respectively. All statistical analyses were conducted in SPSS package 18.0 (SPSS, Chicago, IL).
RESULTS AND DISCUSSION
Concentrations of Urinary OH-PAHs
For the 12 metabolites of PAHs in the subsequent discussion, the detection rates were all greater than 88%. The concentrations of OH-PAHs with different numbers of rings are shown in Figure S1. The median concentrations of hydroxynaphthalenes (ΣOH-NAPs, sum of 1- and 2-OH-NAP), ΣOH-BPs (sum of 2-, 4-OH-BP and 4,4′-DOH-BP), 2-hydroxydibenzofuran (2-OH-DBF), ΣOH-FLUs (sum of 2-, and 3-OH-FLU), ΣOH-PHEs (sum of 1-, 2-, and 4- OH-PHE), and 1-OH-PYR were 4.01, 2.12, 0.60, 0.56, 0.43, and 0.13 μg g−1 creatinine, respectively. A decreasing trend in urinary concentration of OH-PAHs was observed when the number of aromatic rings increased. This was likely because PAHs with fewer aromatic rings tend to present in a higher concentration in the environment and have a higher urine-excretion rate in human body.2,22,23,25,26
The concentration of urinary OH-PAHs was influenced by many factors, such as the environmental levels of PAHs, individual physical activities, and individual characteristics. In this study, the determinants of OH-PAHs were investigated using a multivariate model with stepwise approach based on the questionnaire data, and the results are shown in Table S2. The city (i.e., Los Angeles and Beijing) was the dominant factor determining the urinary OH-PAHs concentrations. Individual characteristics (i.e., gender, age and BMI) were also significant factors (p < 0.05); however, their impacts on the change of OH-PAHs between the two cities were minimized as multiple measurements were conducted for each subject who serves as his or her own control. The individual physical activities, including diet habits and traffic-related activities, differed significantly between the two cities (p < 0.05, Table S3). However, they had limited impacts on the urinary OH-PAHs concentrations in this study as most of them were not significantly associated with OH-PAHs after adjustment for city (Table S2) and thus not considered in the subsequent discussion.
Table 1 shows the difference in the creatinine adjusted OH-PAHs concentration between Los Angles and Beijing (unadjusted data are shown in Table S4). It should be noted that biphenyl and dibenzofuran are technically not PAHs but have similar structure and environment sources with PAHs. Hence in addition to the total concentration of all analytes (Σ12OH-PAHs), the total concentration of metabolites of naphthalene, fluorene, phenanthrene, and pyrene (Σ8OH-PAHs) was also calculated (Table 1). The median concentration of Σ12OH-PAHs in Beijing was 14.1 μg g−1 creatinine, which was significantly higher than that in LA1 (5.77 μg g−1 creatinine, p < 0.001) and LA2 (5.78 μg g−1 creatinine, p < 0.001). No significant difference was observed between the Σ12OH-PAHs concentration in LA1 and LA2 (p = 0.85). A similar trend was also observed for ΣOH-NAPs, ΣOH-BPs, 2-OH-DBF, ΣOH-FLUs, ΣOH-PHEs, and 1-OH-PYR. These results indicate that the observed urinary OH-PAHs levels were mainly driven by the differences of various environmental and activity factors between the two cities. On the basis of these biomarkers, it was estimated that the exposure to different PAHs was 1.3–6.1-fold higher in Beijing than in Los Angeles during the study season (Table 1).
Table 1.
Descriptive Statistics of Biomarkers in Urine Samples in Beijing (BJ) and Los Angeles Before (LA1) and After the Trip (LA2)
| biomarker | median (IQRa) | p valueb | Beijing/LA concentration ratio (95%CI;p value)c | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| in LA1(n = 30) | in BJ(n = 47) | in LA2(n = 27) | LA1 vs BJ | LA2 vs BJ | LA1 vs LA2 | ||
| exposure biomarker (ug/g creatinine) | |||||||
| 1-hydroxynaphthalene | 0.91 (0.35, 1.45) | 1.91 (1.01, 2.62) | 0.41 (0.29, 1.05) | < 0.001 | < 0.001 | 0.08 | 2.6 (1.7–4.0; < 0.001) |
| 2-hydroxynaphthalene | 2.58 (1.13, 4.57) | 2.74 (1.77, 5.00) | 2.43 (1.36, 3.87) | 0.34 | 0.28 | 0.95 | 1.1 (0.79–1.5; 0.67) |
| Σhydroxynaphthalenes | 3.08 (1.85, 5.82) | 5.01 (2.95, 7.94) | 2.76 (1.87, 4.41) | < 0.05 | < 0.05 | 0.57 | 1.3 (0.97–1.8; 0.08) |
| 2-hydroxybiphenyl | 0.45 (0.25, 1.16) | 0.45 (0.24, 1.10) | 0.66 (0.42, 1.46) | 0.87 | 0.19 | 0.20 | 1.1 (0.66–1.7; 0.82) |
| 4-hydroxybiphenyl | 0.30 (0.17, 0.45) | 1.29 (0.85, 1.98) | 0.30 (0.18, 0.43) | < 0.001 | < 0.001 | 0.87 | 3.7 (2.7–5.0; < 0.001) |
| 4,4′-dihydroxybiphenyl | 0.29 (0.18, 0.52) | 0.91 (0.76, 1.29) | 0.29 (0.18, 0.45) | < 0.001 | < 0.001 | 0.89 | 2.7 (2.0–3.7; < 0.001) |
| Σhydroxybiphenyls | 1.39 (0.72, 2.22) | 2.93 (1.84, 4.79) | 1.54 (0.94, 2.77) | < 0.001 | < 0.01 | 0.33 | 2.3 (1.6–3.2; < 0.001) |
| 2-hydroxydibenzofuran | 0.25 (0.14, 0.47) | 1.80 (1.24, 2.07) | 0.25 (0.20, 0.34) | < 0.001 | < 0.001 | 0.60 | 6.1 (4.6–7.9; < 0.001) |
| 2-hydroxyfluorene | 0.20 (0.10, 0.33) | 1.21 (0.80, 1.64) | 0.19 (0.12, 0.25) | < 0.001 | < 0.001 | 0.99 | 5.7 (4.4–7.6; < 0.001) |
| 3-hydroxyfluorene | 0.08 (0.04, 0.14) | 0.41 (0.31, 0.55) | 0.07 (0.04, 0.11) | < 0.001 | < 0.001 | 0.59 | 5.5 (4.0–7.5; < 0.001) |
| Σhydroxyfluorenes | 0.29 (0.13, 0.49) | 1.58 (1.17, 2.31) | 0.26 (0.17, 0.34) | < 0.001 | < 0.001 | 0.91 | 5.6 (4.3–7.4; < 0.001) |
| 1-hydroxyphenanthrene | 0.12 (0.06, 0.21) | 0.41 (0.30, 0.73) | 0.08 (0.06, 0.17) | < 0.001 | < 0.001 | 0.29 | 3.9 (2.9–5.2; < 0.001) |
| 2-hydroxyphenanthrene | 0.08 (0.04, 0.13) | 0.26 (0.15, 0.36) | 0.06 (0.04, 0.12) | < 0.001 | < 0.001 | 0.53 | 3.5 (2.7–4.6; < 0.001) |
| 4-hydroxyphenanthrene | 0.04 (0.02, 0.07) | 0.12 (0.07, 0.20) | 0.04 (0.02, 0.10) | < 0.001 | < 0.001 | 0.87 | 2.7 (1.9–3.9; < 0.001) |
| Σhydroxyphenanthrenes | 0.25 (0.15, 0.43) | 0.92 (0.52, 1.26) | 0.20 (0.13, 0.36) | < 0.001 | < 0.001 | 0.48 | 3.5 (2.6–4.6; < 0.001) |
| 1-hydroxypyrene | 0.09 (0.05, 0.16) | 0.32 (0.18, 0.46) | 0.07 (0.05, 0.13) | < 0.001 | < 0.001 | 0.99 | 3.3 (2.4–4.6; < 0.001) |
| Σ8hydroxylated PAHsd | 3.76 (2.16, 7.06) | 8.85 (4.99, 12.1) | 3.27 (2.37, 5.54) | < 0.01 | < 0.001 | 0.62 | 1.8 (1.3–2.4; < 0.001) |
| Σ12hydroxylated PAHse | 5.77 (3.63, 10.6) | 14.1 (7.68, 20.5) | 5.78 (3.70, 10.5) | < 0.001 | < 0.001 | 0.85 | 2.0 (1.5–2.7; < 0.001) |
| metabolite ratio | |||||||
| 1/2-hydroxynaphthalene | 0.29 (0.13, 0.55) | 0.53 (0.34, 0.96) | 0.15 (0.11, 0.34) | < 0.01 | < 0.001 | 0.14 | 2.5 (1.7–3.5; < 0.001) |
| 1 + 2/4-hydroxyphenanthrene | 3.99 (2.88, 7.48) | 5.08 (4.15, 8.15) | 4.00 (2.91, 5.83) | 0.07 | < 0.01 | 0.46 | 1.4 (1.1–1.7; < 0.01) |
| effect biomarker (ug/g creatinine) | |||||||
| malondialdehyde | 53.7(36.6, 72.5) | 48.4 (39.4, 68.3) | 49.6 (38.4, 90.8) | 0.75 | 0.40 | 0.78 | 0.93 (0.77–1.2; 0.46) |
IQR: interquartile range.
Mann–Whitney test.
Ratio =10β, where β is the estimated slope for city in multivariate linear regression models with the enter approach and OH-PAHs is log-transformed. Ratios are adjusted by age, gender, and BMI.
Sum of hydroxynaphthalenes, hydroxyfluorenes, hydroxyphenanthrenes, and 1-hydroxypyrene.
Sum of hydroxynaphthalenes, hydroxybiphenyls, 2-hydroxydibenzofuran, hydroxyfluorenes, hydroxyphenanthrenes, and 1-hydroxypyrene.
As a classic biomarker for PAHs exposure, 1-OH-PYR is widely measured in populations around the world.27,28 Hence, it was used for comparison with other studies. As shown in Figure S2, the concentration of 1-OH-PYR in Beijing (median, 0.32 μg g−1 creatinine) was higher than that of most cities in developed countries, such as San Francisco, United States (0.08 μg g−1 creatinine)22 and Christchurch, New Zealand (0.04 μg g−1 creatinine),29 but lower than that of most cities in developing countries, such as Nanjing, China (1.08 μg g−1 creatinine)30 and Bangkok, Thailand (0.39 μg g−1 creatinine).31 The concentration of 1-OH-PYR in Los Angeles (0.08 μg g−1 creatinine) was comparable to that in cities in developed countries. Those comparisons indicated that the exposure to PAHs in the summer in both Beijing and Los Angeles was at an intermediate level worldwide.
Difference in the Ratios of OH-PAH Isomers
As discussed above, ΣOH-NAPs and ΣOH-BPs differed significantly in the two cities; however, not all metabolites from the same precursor PAHs showed the same concentration ratios between the two cities (Table 1). Briefly, the concentration ratios of 1-OH-NAP, 4-OH-BP, and 4,4′-DOH-BP between Beijing and Los Angeles were significantly greater than 1.0 (p < 0.001). In contrast, the concentration ratios of 2-OH-NAP and 2-OH-BP were not significantly different with 1.0. The difference of these OH-PAH isomers indicated potential bias may exist if only one or few isomers were used as surrogates for total PAHs exposures. Instead, the sum of OH-PAHs isomers (i.e., ΣOH-NAPs and ΣOH-BPs) could be the least-biased surrogate for PAHs exposure given the concentrations of multiple OH-PAHs are available.
The reason for the different concentration ratios of OH-PAH isomers between the two cities is unclear, and the interaction between PAHs and cytochrome P450 (CYP) enzymes may be a possible mechanism. PAHs could be metabolized by a series of CYP enzymes, such as CYP1A1 and CYP1B1, through an arene oxide intermediate to form hydroxylated metabolites.32 Different CYPs in the phase I metabolism of PAHs could result in different metabolite (i.e., OH-PAHs) ratios.33–35 Meanwhile, PAHs and their metabolites could in turn induce or inhibit the expression of CYPs, which could alter the profiles of CYP enzymes involved in the metabolism of PAHs and then further alter the ratios of OH-PAHs isomers.33,36 In this study, a higher exposure to PAHs was observed in Beijing, which could possibly cause a shift in the relative expression of different CYPs and might therefore lead to a corresponding shift in the OH-PAHs isomer ratios.
Because previous studies revealed a difference in PAHs metabolite ratios under the catalysis of different CYPs,33–35 we suspect there may be a link between the alteration of OH-PAHs isomer ratios and the exposure-induced alteration of CYPs expression. To test this hypothesis, we investigated the difference in several OH-PAHs isomer ratios between the two cities. First, the ratio of 1-OH-NAP to 2-OH-NAP (1-/2-OH-NAP) was investigated because (1) 1-OH-NAP and 2-OH-NAP were the only monohydroxylated metabolites of naphthalene so that the ratio would not be influenced by other monohydroxylated metabolites; and (2) the 1-/2-OH-NAP was mathematically independent from the ΣOH-NAPs. As expected, the 1-/2-OH-NAP ratio was significantly elevated in Beijing, suggesting a possible shift in the relative expression of CYPs. It should be noted that the elevation of 1-/2-OH-NAP occurred gradually after the students arrived in Beijing (Figure 1), possibly suggesting the alteration of metabolism could be a subacute process. This may explain the observation in other studies that the variation of OH-PAHs isomer concentrations tended to be more consistent after an accidental high exposure.23,37
It should be noted that 1-OH-NAP is also a metabolite of carbaryl pesticides.38 If 1-/2-OH-NAP was influenced by carbaryl pesticides exposure, we would expect that 1-/2-OH-NAP had a more significant association with ΣOH-NAPs than with other OH-PAHs. However, as shown in Table S5, 1-/2-OH-NAP was not correlated with ΣOH-NAPs but significantly correlated with other OH-PAHs, indicating carbaryl pesticides have limited impacts in this study.
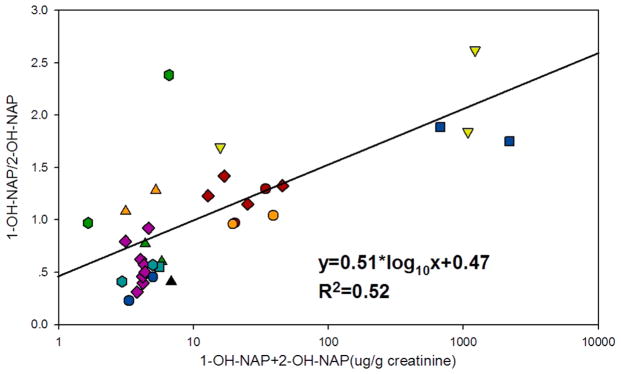
To further confirm the relationship between the 1-/2-OH-NAP ratio and PAHs exposure, we conducted an analysis on selected literature. The selection criteria includes: (1) the population was under a well-defined long-term exposure; (2) the PAHs to which the population was exposed were mainly from combustion sources to minimize the interference from carbaryl pesticide; and (3) the sample size is larger than 10 to decrease the uncertainty caused by individual difference. Because the number and species of the OH-PAHs measured varied among different studies, the concentration of ΣOH-NAPs was used as an indicator for total exposure to PAHs. The results and the description of the literature searches are shown in Figure 2 and Table S6. A significant association was observed between the 1-/2-OH-NAP and ΣOH-NAPs (R2 = 0.52, p < 0.001). Additionally, for studies in which repeated measurements were conducted that minimized the genetic factors, an interstudy relationship between 1-/2-OH-NAP and ΣOH-NAPs was also observed.39–44 These results revealed a potential shift in the relative expression of CYPs that might be related to PAH exposures.
Figure 2.
The association between naphthalene exposure and 1-/2-OH-NAP ratio. Red circle, cooking women;41 orange circle, cooking women;40 light green downward-facing triangle, coking workers;52 dark green upward-facing triangle, road construction workers;43 teal square, healthy general people;30 dark blue square, coking workers;53 purple diamond, general people near an aluminum plant;39 red diamond, coking workers;6 orange upward-facing triangle, general people near a creosote impregnation plant;44 black upward-facing triangle, schoolchildren near a road;54 dark green hexagon, brick kiln workers;55 teal hexagon, U.S. air forces personnel;42 blue hexagon, this study. The detailed information on these studies is shown in Table S6.
The alteration of 1-/2-OH-NAP could explain why 1-OH-NAP and 2-OH-NAP had different concentration ratios between the two cities (Table 1). For 1-OH-NAP, the exposure to PAHs and the corresponding alteration of 1-/2-OH-NAP were in the same direction; therefore, the concentration of 1-OH-NAP was significantly higher in Beijing. However, for 2-OH-NAP, the change in exposure to PAHs could be offset by alterations in the ratio; thus, the concentration of 2-OH-NAP was observed to be similar in the two cities.
This mechanism could also explain the observation of OH-PHEs isomers. Previous studies have shown that 1-OH-PHE and 2-OH-PHE are mainly derived from the same CYPs (e.g., CYP1A1), while 3-OH-PHE and 4-OH-PHE come from other CYPs (e.g., CYP1A2).22,34,45 These findings were consistent with the observations in this study that the 1 + 2-/4-OH-PHE ratio was significantly elevated in Beijing (p < 0.01, Table 1). In addition, 1 + 2-/4-OH-PHE was significantly correlated with several OH-PAHs (i.e., 2-OH-DBF and ΣOH-FLU, p < 0.05, Table S5), possibly suggesting a similar link between exposure and metabolism.
Previous studies found that smoking could decrease 1 + 2-/3 + 4-OH-PHE,22 suggesting exposure to secondhand smoke (SHS) may reduce the 1 + 2-/4-OH-PHE. In our study, SHS exposure is significantly higher in Beijing (Table S3). To distinguish the impacts of PAHs exposure from SHS and non-SHS sources, we divided the data in Beijing into two groups. As shown in Figure S3, all subjects in Beijing had significantly higher ΣOH-PHEs and 1 + 2-/4-OH-PHE than in Los Angeles. Subjects in Beijing with SHS exposures tend to have slightly higher ΣOH-PHEs but lower 1 + 2-/4-OH-PHE compared with those without SHS exposures, which was consistent with previous studies on smoking.22 However, no significant difference was observed between subjects with and without SHS exposures in Beijing. These results indicate that the elevation of ΣOH-PHEs and 1 + 2-/4-OH-PHE in Beijing was probably attributed to sources other than SHS.
Association between MDA and OH-PAHs
MDA was a product of lipid oxidative damage and hence was used as an indicator of lipid peroxidation.15 In this study, MDA was detected in all the urine samples, and their median concentrations were 48.4 and 51.9 μg g−1 creatinine in Beijing and Los Angeles, respectively. No significant difference in the concentration of MDA was observed between the two cities (Figure 1 and Table 1). The relationship between MDA and OH-PAHs is shown in Figure S4. MDA was significantly correlated with Σ12OH-PAHs (p < 0.05); however, for speciation analysis, only ΣOH-BPs was significantly correlated with MDA (p < 0.05). This result is out of our expectation because most species measured in this study were found to strongly associate with MDA or other oxidative damage biomarkers (i.e., 8-hydroxy-2-deoxyguanosine and 8-iso-prosta-glandin-F2α) as shown in previous studies.6,14,46,47 In addition, the association between MDA and several OH-PAHs is marginally significant (Figure S4), suggesting there are some interference factors affecting the association.
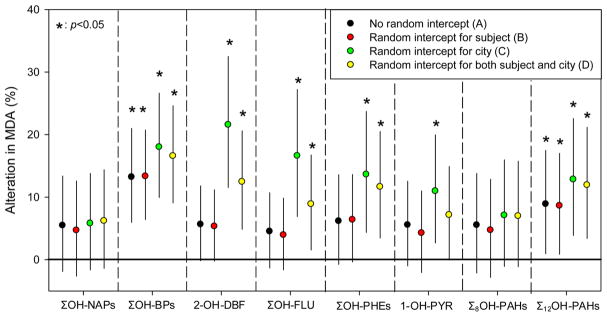
To investigate the possible interference factors, we applied a simple linear regression model (Model A) and three mixed-effects models (Models B, C, and D) to study the association between MDA and OH-PAHs and then compared the results of different models. In Model A, the association between OH-PAHs and MDA was considered constant among individuals and cities, which is corresponding to the results in Figure S4. Among six OH-PAHs homologues, only ΣOH-BPs is significantly associated with MDA (p < 0.05). In Model B, the intercept was allowed to vary among subjects. As shown in Figure 3, the association between OH-PAHs and MDA was comparable with that in Model A, indicating that individual difference did not cause a significant interference in this study. In Model C, the intercept was allowed to vary between the two cities, and the results revealed a significant association between MDA and all OH-PAHs except for ΣOH-NAPs. Compared with Model A, the association between OH-PAHs and MDA was generally more significant, indicating that city is a major interference factor. The results of Model D, in which the intercept was varied among both subjects and cities, were similar to that of Model C, once again indicating a limited impact of individual difference compared with city.
Figure 3.
Association between OH-PAHs and MDA. The alteration in MDA (%) was associated with a one-fold increase of OH-PAHs. Σ8OH-PAHs: sum of ΣOH-NAPs, ΣOH-FLUs, ΣOH-PHEs, and 1-OH-PYR; Σ12OH-PAHs: sum of Σ8OH-PAHs, ΣOH-BPs, and 2-OH-DBF.
As discussed above, the association between OH-PAHs and MDA was found to vary between the two cities, even for the same subject. There are several possible explanations for the observed city effect on associations: (1) the exposure to PAHs could induce the change in antioxidants in the human body,48 which could affect an individual’s oxidative stress; (2) the urinary MDA concentration was affected by other factors differing in two cities, such as the diet intake of MDA precursors and the decomposition conditions of MDA;49 and (3) the cities’ differences in the concentration of other pollutants that could induce oxidative damage15 may interfere with the association between MDA and OH-PAHs. However, the potential mechanism of the observed city-effect is beyond the scope of this study and calls for future studies. Nevertheless, it is important to address that the associations between MDA and OH-PAHs are generally significant only if the city effect was considered. This is probably because OH-PAHs were significantly higher in Beijing but MDA was comparable between the two cities, which could weaken the inter-city associations (Figure S5).
There are several limitations of this study. First, the external exposures to PAHs were not measured, and thus, the OH-PAHs results could not be attributed to specific sources. For example, the time spent in indoor environments was not assessed in this study but may be an important factor affecting PAHs exposure levels and the related health effects.50,51 Second, many factors (e.g., diet, stress, and physical activities, etc.) may have changed when the subjects traveled from Los Angeles to Beijing. How these factors affect OH-PAHs measured in this study is not fully understood. Finally, because the CYP enzyme cannot be readily measured in human subjects, how it affects the observed difference of the ratios of some OH-PAH isomers between the two cities cannot be determined.
In summary, this study identified significantly higher PAHs exposure and homogeneous OH-PAHs ratios in Beijing compared with Los Angeles in summer 2012. It also found a significant association between PAHs exposure and lipid peroxidation, with the association varying between the two cities. This study highlighted a possible link between PAH exposure and metabolism that needs to be considered in future health-effect studies.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (NIEHS, 1R21ES024560) and the National Natural Science Foundation of China (21322705, 41421064, and 21190051), the Top-Notch Young Talents Program of China, the Collaborative Innovation Center for Regional Environmental Quality, and the Chinese Scholarship Council.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.5b04629.
Tables showing information of OH-PAHs standards, the monitored ions of methylated OH-PAHs in GC-MS (EI) and the method detection limits of OH-PAHs, the influence of individual characteristic and physical activities on urinary OH-PAHs based on multivariate regression model with stepwise approach, comparison between the physical activities in Los Angeles and Beijing, descriptive statistics of biomarkers urine samples in Beijing (BJ) and Los Angeles before (LA1) and after the trip (LA2), Pearson correlation among different OH-PAHs and metabolite ratios, and summary of the studies cited to investigate the relationship between naphthalene exposure and 1-/2-OH-NAP ratio. Figures showing the concentration of grouped urinary OH-PAHs; concentration of urinary 1-OH-PYR in the population around world; a comparison in 1+2/4-PHEs, OH-PHEs, and time in secondhand smoke (SHS) among population in Los Angeles, in Beijing without SHS exposure, and in Beijing with SHS; correlation between MDA and OH-PAHs in both cities; and correlation between MDA and 1-OH-PYR in Los Angeles and Beijing. (PDF)
References
- 1.Shen H, Huang Y, Wang R, Zhu D, Li W, Shen G, Wang B, Zhang Y, Chen Y, Lu Y. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ Sci Technol. 2013;47(12):6415–6424. doi: 10.1021/es400857z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K, Jahan SA, Kabir E, Brown RJ. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Shi S, Zhao B. Modeled Exposure Assessment via Inhalation and Dermal Pathways to Airborne Semivolatile Organic Compounds (SVOCs) in Residences. Environ Sci Technol. 2014;48(10):5691–5699. doi: 10.1021/es500235q. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Cause Control. 1997;8(3):444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 5.Deng Q, Huang S, Zhang X, Zhang W, Feng J, Wang T, Hu D, Guan L, Li J, Dai X, Deng H, Zhang X, Wu T. Plasma microRNA Expression and Micronuclei Frequency in Workers Exposed to Polycyclic Aromatic Hydrocarbons. Environ Health Persp. 2014;122(7):719–725. doi: 10.1289/ehp.1307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuang D, Zhang W, Deng Q, Zhang X, Huang K, Guan L, Hu D, Wu T, Guo H. Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol. 2013;47(13):7446–7456. doi: 10.1021/es401639x. [DOI] [PubMed] [Google Scholar]
- 7.Padula AM, Balmes JR, Eisen EA, Mann J, Noth EM, Lurmann FW, Pratt B, Tager IB, Nadeau K, Hammond SK. Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. J Exposure Sci Environ Epidemiol. 2015;25(3):295–302. doi: 10.1038/jes.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Feng Y, Deng H, Zhang W, Kuang D, Deng Q, Dai X, Lin D, Huang S, Xin L, et al. The Dose–Response Decrease in Heart Rate Variability: Any Association with the Metabolites of Polycyclic Aromatic Hydrocarbons in Coke Oven Workers? PLoS One. 2012;9(7):e44562. doi: 10.1371/journal.pone.0044562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Zhou Y, Sun H, Lai H, Liu C, Yan K, Yuan J, Wu T, Chen W, Zhang X. Dose-response relationship between polycyclic aromatic hydrocarbon metabolites and risk of diabetes in the general Chinese population. Environ Pollut. 2014;195:24–30. doi: 10.1016/j.envpol.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang Le, Zhu H, Finnell RH, Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A. 2011;108(31):12770–12775. doi: 10.1073/pnas.1105209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Cook RL, Ilacqua VA, Kan H, Talbott EO, Kearney G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci Total Environ. 2010;408(21):4943–4948. doi: 10.1016/j.scitotenv.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung Cancer Risk after Exposure to Polycyclic Aromatic Hydrocarbons: A Review and Meta-Analysis. Environ Health Persp. 2004;112(9):970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Yang Q, Qiu X, Li K, Li G, Zhu P, Zhu T. Reactive Oxygen Species Alteration of Immune Cells in Local Residents at an Electronic Waste Recycling Site in Northern China. Environ Sci Technol. 2013;47(7):3344–3352. doi: 10.1021/es400027v. [DOI] [PubMed] [Google Scholar]
- 14.Yang QY, Qiu XH, Li R, Ma J, Li KQ, Li G. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environ Sci Pollut Res. 2015;22(3):1760–1769. doi: 10.1007/s11356-014-3284-y. [DOI] [PubMed] [Google Scholar]
- 15.Bae S, Pan X, Kim S, Park K, Kim Y, Kim H, Hong Y. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Persp. 2010;118(4):579. doi: 10.1289/ehp.0901077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polidori MC, Savino K, Alunni G, Freddio M, Senin U, Sies H, Stahl W, Mecocci P. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity. Free Radical Biol Med. 2002;32(2):148–152. doi: 10.1016/s0891-5849(01)00782-1. [DOI] [PubMed] [Google Scholar]
- 17.Huang R, Zhang Y, Bozzetti C, Ho K, Cao J, Han Y, Daellenbach KR, Slowik JG, Platt SM, Canonaco F, et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature. 2014;514(7521):218–222. doi: 10.1038/nature13774. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Ma Y, Qiu X, Li R, Fang Y, Wang J, Zhu Y, Hu D. Sources, transformation, and health implications of PAHs and their nitrated, hydroxylated, and oxygenated derivatives in PM2.5 in Beijing. J Geophys Res. 2015;120(14):7219–7228. [Google Scholar]
- 19.Lin Y, Qiu X, Ma Y, Ma J, Zheng M, Shao M. Concentrations and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in the atmosphere of North China, and the transformation from PAHs to NPAHs. Environ Pollut. 2015;196:164–170. doi: 10.1016/j.envpol.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu T, Dashwood RH, Zhang W, Wang X, Simonich SLM. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ Sci Technol. 2011;45(16):6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thai PK, Li Z, Sjödin A, Fox A, Diep NB, Binh TT, Mueller JF. Biomonitoring of polycyclic aromatic hydrocarbons exposure in small groups of residents in Brisbane, Australia and Hanoi, Vietnam, and those travelling between the two cities. Chemosphere. 2015;139:358–364. doi: 10.1016/j.chemosphere.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StHelen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, III, Benowitz NL. Exposure and Kinetics of Polycyclic Aromatic Hydrocarbons (PAHs) in Cigarette Smokers. Chem Res Toxicol. 2012;25(4):952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol. 2012;25(7):1452–1461. doi: 10.1021/tx300108e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer T, Scheline RR. The Metabolism of Biphenyl. II. Phenolic Metabolites in the Rat. Acta Pharmacol Toxicol. 1976;39(4):419–432. doi: 10.1111/j.1600-0773.1976.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 25.Marie C, Bouchard M, Heredia Ortiz R, Viau C, Maitre A. A toxicokinetic study to elucidate 3-hydroxybenzo (a) pyrene atypical urinary excretion profile following intravenous injection of benzo (a) pyrene in rats. J Appl Toxicol. 2010;30(5):402–410. doi: 10.1002/jat.1511. [DOI] [PubMed] [Google Scholar]
- 26.Viau C, Bouchard M, Carrier G, Brunet R, Krishnan K. The toxicokinetics of pyrene and its metabolites in rats. Toxicol Lett. 1999;108(2):201–207. doi: 10.1016/s0378-4274(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 27.Tsai P, Shih T, Chen H, Lee W, Lai C, Liou S. Urinary 1-Hydroxypyrene as an Indicator for Assessing the Exposures of Booth Attendants of a Highway Toll Station to Polycyclic Aromatic Hydrocarbons. Environ Sci Technol. 2004;38(1):56–61. doi: 10.1021/es030588k. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Senthilkumar K, Alomirah H, Moon HB, Minh TB, Mohd MA, Nakata H, Kannan K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013;47(6):2932–8. doi: 10.1021/es3052262. [DOI] [PubMed] [Google Scholar]
- 29.Cavanagh JE, Brown L, Trought K, Kingham S, Epton MJ. Elevated concentrations of 1-hydroxypyrene in schoolchildren during winter in Christchurch, New Zealand. Sci Total Environ. 2007;374(1):51–59. doi: 10.1016/j.scitotenv.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Zhu P, Han Y, Lu C, Wang S, Gu A, Fu G, Zhao R, Song L, Wang X. Urinary metabolites of polycyclic aromatic hydrocarbons in relation to idiopathic male infertility. Hum Reprod. 2009;24(5):1067–1074. doi: 10.1093/humrep/dep006. [DOI] [PubMed] [Google Scholar]
- 31.Ruchirawat M, Settachan D, Navasumrit P, Tuntawiroon J, Autrup H. Assessment of potential cancer risk in children exposed to urban air pollution in Bangkok, Thailand. Toxicol Lett. 2007;168(3):200–209. doi: 10.1016/j.toxlet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21(4):257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 33.Spink DC, Wu SJ, Spink BC, Hussain MM, Vakharia DD, Pentecost BT, Kaminsky LS. Induction of CYP1A1 and CYP1B1 by benzo (k) fluoranthene and benzo (a) pyrene in T-47D human breast cancer cells: Roles of PAH interactions and PAH metabolites. Toxicol Appl Pharmacol. 2008;226(3):213–224. doi: 10.1016/j.taap.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob JUR, Raab G, Soballa V, Schmalix WA, Grimmer G, Greim H, Doehmer J, Seidel A. Cytochrome P450-mediated activation of phenanthrene in genetically engineered V79 Chinese hamster cells. Environ Toxicol Pharmacol. 1996;1(1):1–11. doi: 10.1016/1382-6689(95)00003-8. [DOI] [PubMed] [Google Scholar]
- 35.Cho TM, Rose RL, Hodgson E. In vitro metabolism of naphthalene by human liver microsomal P450 enzymes. Drug Metab Dispos. 2006;34(1):176–183. doi: 10.1124/dmd.105.005785. [DOI] [PubMed] [Google Scholar]
- 36.Shimada T, Murayama N, Okada K, Funae Y, Yamazaki H, Guengerich FP. Different mechanisms for inhibition of human cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic inhibitors. Chem Res Toxicol. 2007;20(3):489–496. doi: 10.1021/tx600299p. [DOI] [PubMed] [Google Scholar]
- 37.Motorykin O, Santiago-Delgado L, Rohlman D, Schrlau JE, Harper B, Harris S, Harding A, Kile ML, Massey Simonich SLM. Metabolism and excretion rates of parent and hydroxy-PAHs in urine collected after consumption of traditionally smoked salmon for Native American volunteers. Sci Total Environ. 2015;514:170–177. doi: 10.1016/j.scitotenv.2015.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J Exposure Sci Environ Epidemiol. 2007;17(4):314–320. doi: 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- 39.Bouchard M, Normandin L, Gagnon F, Viau C, Dumas P, Gaudreau ER, Tremblay C. Repeated measures of validated and novel biomarkers of exposure to polycyclic aromatic hydrocarbons in individuals living near an aluminum plant in Quebec, Canada. J Toxicol Environ Health, Part A. 2009;72(23):1534–1549. doi: 10.1080/15287390903129481. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Sj O, Din A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int. 2011;37(7):1157–1163. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, Masera O, Li Z, Romanoff L, Sj O Din A, Rojas-Bracho L, Needham LL, Romieu I. Impact of the improved patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural mexican women. Environ Health Persp. 2011;119(9):1301. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues EG, Smith K, Maule AL, Sjodin A, Li Z, Romanoff L, Kelsey K, Proctor S, McClean MD. Urinary Polycyclic Aromatic Hydrocarbon (OH-PAH) Metabolite Concentrations and the Effect of GST Polymorphisms Among US Air Force Personnel Exposed to Jet Fuel. J Occup Environ Med. 2014;56(5):465–471. doi: 10.1097/JOM.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pesch B, Spickenheuer A, Kendzia B, Schindler BK, Welge P, Marczynski B, Rihs H, Raulf-Heimsoth M, Angerer J, Brüning T. Urinary metabolites of polycyclic aromatic hydrocarbons in workers exposed to vapours and aerosols of bitumen. Arch Toxicol. 2011;85(1):29–39. doi: 10.1007/s00204-011-0680-7. [DOI] [PubMed] [Google Scholar]
- 44.Bouchard MEL, Pinsonneault L, Tremblay C, Weber J. Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons in subjects living in the vicinity of a creosote impregnation plant. Int Arch Occup Environ Health. 2001;74(7):505–513. doi: 10.1007/s004200100251. [DOI] [PubMed] [Google Scholar]
- 45.Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4(5):319–327. doi: 10.1080/135475099230705. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Han I, Hu M, Shao M, Zhang JJ, Tang X. Personal exposure to particulate PAHs and anthraquinone and oxidative DNA damages in humans. Chemosphere. 2010;81(10):1280–1285. doi: 10.1016/j.chemosphere.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 47.Deng Q, Dai X, Guo H, Huang S, Kuang D, Feng J, Wang T, Zhang W, Huang K, Hu D, Deng H, Zhang X, Wu T. Polycyclic Aromatic Hydrocarbons-Associated MicroRNAs and Their Interactions with the Environment: Influences on Oxidative DNA Damage and Lipid Peroxidation in Coke Oven Workers. Environ Sci Technol. 2014;48(7):4120–4128. doi: 10.1021/es4055516. [DOI] [PubMed] [Google Scholar]
- 48.Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res, Genet Toxicol Environ Mutagen. 2009;674(1–2):137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102(5):589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou B, Zhao B. Population inhalation exposure to polycyclic aromatic hydrocarbons and associated lung cancer risk in Beijing region: Contributions of indoor and outdoor sources and exposures. Atmos Environ. 2012;62:472–480. [Google Scholar]
- 51.Chen C, Zhao B, Weschler CJ. Indoor Exposure to “Outdoor PM10”: Assessing Its Influence on the Relationship Between PM10 and Short-term Mortality in U.S. Cities. Epidemiology. 2012;23(6):870. doi: 10.1097/EDE.0b013e31826b800e. [DOI] [PubMed] [Google Scholar]
- 52.Bieniek G, et al. Urinary naphthols as an indicator of exposure to naphthalene. Scand J Work, Environ Health. 1997;23(6):414–420. doi: 10.5271/sjweh.263. [DOI] [PubMed] [Google Scholar]
- 53.Bieniek G. Aromatic and polycyclic hydrocarbons in air and their urinary metabolites in coke plant workers. Am J Ind Med. 1998;34(5):445–454. doi: 10.1002/(sici)1097-0274(199811)34:5<445::aid-ajim5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Barraza-Villarreal A, Escamilla-Nuñez MC, Schilmann A, Hernandez-Cadena L, Li Z, Romanoff L, Sjödin A, Del Río-Navarro BE, Díaz-Sanchez D, Díaz-Barriga F, Sly P, Romieu L. Lung Function, Airway Inflammation, and Polycyclic Aromatic Hydrocarbons Exposure in Mexican Schoolchildren: A Pilot Study. J Occup Environ Med. 2014;56(4):415–419. doi: 10.1097/JOM.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamal A, Malik RN, Martellini T, Cincinelli A. PAH exposure biomarkers are associated with clinico-chemical changes in the brick kiln workers in Pakistan. Sci Total Environ. 2014;490:521–527. doi: 10.1016/j.scitotenv.2014.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.