Figure 3.
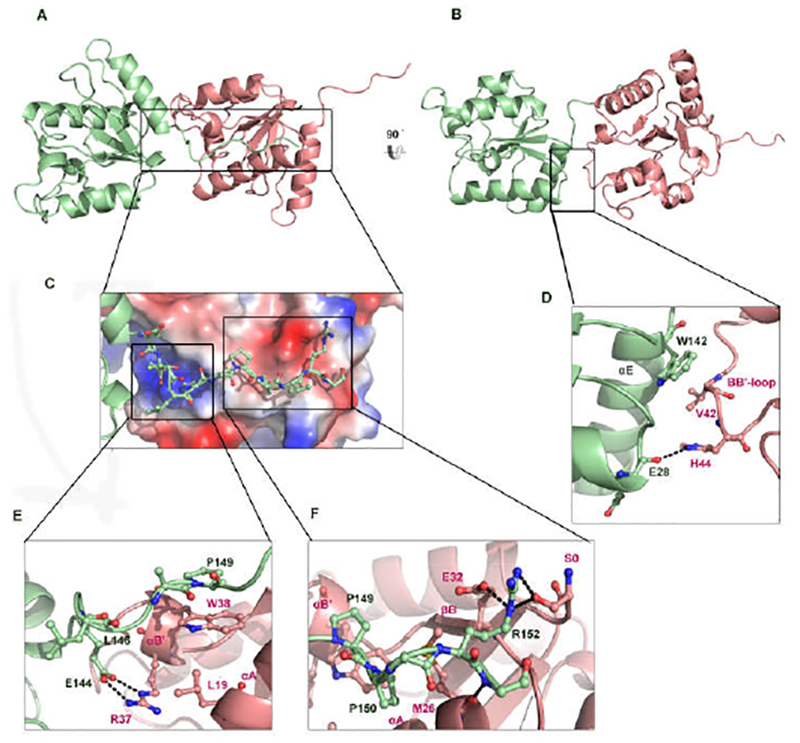
The interface formed by the protruding tail of BcSEFIR. (A) In this asymmetric dimer of BcSEFIR, the tail-presenting molecule is colored in light green, as wellas the tail-docking one in light pink. Helices αA, αB, αB′ are involved in the interaction. Among them, αB, αB′ play the most important role. Interface formed by thetail portion is highlighted with boxes. (B) The asymmetric dimer is shown in different orientation, interface is highlighted with a box. (C) The zoomed-in view of the protruding tail attaching to the adjacent BcSEFIR molecule. (D) Detailed interactions of the αE and αA helices in the tail-presenting BcSEFIR with the αB in the taildocking BcSEFIR. (E) Detailed interactions of the N-terminal tail and intermediate portion in the tail-presenting BcSEFIR with the αA, αB′ helices in the tail-docking BcSEFIR. Interacting residues are labeled, hydrogen-bonds are shown as dashed lines. (F) Detailed interactions of the tail C-terminal portion in the tail-presenting BcSEFIR with the αA, αB′ helices and βB in the tail-docking BcSEFIR, where interacting residues are labeled, and hydrogen-bonds are shown as dashed lines.
