Abstract
Infant gross motor development is vital to adaptive function and predictive of both cognitive outcomes and neurodevelopmental disorders. However, little is known about neural systems underlying the emergence of walking and general gross motor abilities. Using resting state fcMRI, we identified functional brain networks associated with walking and gross motor scores in a mixed cross-sectional and longitudinal cohort of infants at high and low risk for autism spectrum disorder, who represent a dimensionally distributed range of motor function. At age 12 months, functional connectivity of motor and default mode networks was correlated with walking, whereas dorsal attention and posterior cingulo-opercular networks were implicated at age 24 months. Analyses of general gross motor function also revealed involvement of motor and default mode networks at 12 and 24 months, with dorsal attention, cingulo-opercular, frontoparietal, and subcortical networks additionally implicated at 24 months. These findings suggest that changes in network-level brain–behavior relationships underlie the emergence and consolidation of walking and gross motor abilities in the toddler period. This initial description of network substrates of early gross motor development may inform hypotheses regarding neural systems contributing to typical and atypical motor outcomes, as well as neurodevelopmental disorders associated with motor dysfunction.
Keywords: functional connectivity, gross motor, infant, network, walking
Introduction
Gross motor behavior is one of the earliest directly observable elements of adaptive function (Gibson and Pick 2000). The emergence of walking, for example, signals an infant’s growing autonomy and expanded opportunities for environmental exploration, perceptual learning, and social interaction (Campos et al. 2000; Gibson and Pick 2000). Although detailed behavioral characterization of gross motor development in infants and toddlers has contributed to clinically useful behavioral measures (Mullen 1995), studies of the neural correlates of early gross motor abilities are substantially more limited, particularly given the methodological challenges of task-based imaging in infants. The neurobiology underlying motor development can provide important insights into mechanisms of cognitive development, as motor learning requires multiple cognitive operations, including perception and action planning (von Hofsten 2004; Leisman et al. 2016). Further, brain regions supporting motor function, such as the cerebellum (Dosenbach et al. 2007) and dorsolateral prefrontal cortex (Niendam et al. 2012), are also implicated in cognitive control (Diamond 2000), and early motor development correlates with later cognitive outcomes, including academic achievement and executive function (Murray et al. 2006; Bornstein et al. 2013; Ghassabian et al. 2016). Gross motor dysfunction, in turn, is associated with several neurodevelopmental disorders involving deficits in cognition, including autism spectrum disorder (ASD) (Fournier et al. 2010), attention deficit/hyperactivity disorder (Kaiser et al. 2014), and language disorder (Hill 2001). In the case of ASD, gross motor delays, such as infant head lag (Flanagan et al. 2012), and lower overall gross motor function (Lloyd et al. 2013; Estes et al. 2015), are among the earliest risk markers for subsequent diagnosis. Therefore, elucidating the relationship between key aspects of early gross motor development and brain function will not only inform future research on neural systems contributing to typical cognitive development, but may also guide novel interventions targeting atypical development.
In the past decade, tremendous progress has been made in the application of resting state functional connectivity MRI (fcMRI) to studies of brain development in infancy (for review, see Graham et al. 2015; Gao et al. 2016). By quantifying the correlated spontaneous fluctuations in the blood oxygen level dependent (BOLD) signal across the entire brain, fcMRI allows investigation of the functional architecture of neural systems (Biswal et al. 1995). Infant fcMRI studies have demonstrated that functional networks are readily identifiable in infancy (Fransson et al. 2011; Smyser et al. 2011; Gao et al. 2015a) and have begun to characterize early maturational profiles of several networks, including the sensorimotor network (Gao et al. 2015a). In older children and adults, fc within the motor network, as well as connectivity across networks, has been implicated in motor performance (Barber et al. 2012; Seidler et al. 2015), and interregional and intraregional alterations in fc have been linked to motor deficits in ASD (Nebel et al. 2014b; Carper et al. 2015; Khan et al. 2015). However, it is unknown whether the emergence and advancement of gross motor skills in early development is correlated with connectivity within and across networks, and if so, which networks are strongly involved. The application of network-based analyses to fcMRI thus provides an important avenue for clarifying functional neural systems underlying early gross motor behavior, as well as the developmental course of these brain–behavior relationships.
To characterize relationships between functional brain architecture and the development of walking and general gross motor ability, we examined data from the Infant Brain Imaging Study (IBIS), a longitudinal, prospective study of brain and behavior development in infants at high and low familial risk for ASD. Because children at high risk for ASD also exhibit more developmental delays (Messinger et al. 2013), this unique sample, which included longitudinal and cross-sectional subjects to maximize experimental power, provided sufficient variation in motor ability to detect evolving brain–behavior relationships. Scores for walking and gross motor function were analyzed in relation to network-level fc based on fcMRI data acquired from infants and toddlers during natural sleep. We adapted enrichment analysis, a data-driven statistical method from genetic association studies (Rivals et al. 2007; Backes et al. 2014; Khatri et al. 2012), which afforded a brain-wide approach to identify networks with a significantly increased density of connections strongly related to the studied behaviors. We hypothesized that (1) this strategy would allow identification of specific brain–behavior relationships for walking and general gross motor function, (2) motor network(s) would be engaged in several of these network-level relationships, and (3) differences in network-level relationships would be identified between 12 and 24 months, in line with the rapid progression of walking and gross motor skills during this epoch.
Materials and Methods
Participants
IBIS is a longitudinal multisite (National Institute of Health-funded Autism Centers of Excellence Network) imaging study of infants at increased familial risk for ASD (high-risk infants: HR), defined as such by virtue of having an older sibling with a diagnosis of ASD, together with a low-risk comparison group of infant siblings with no family history of ASD (low-risk: LR) (see Hazlett et al. 2012; Wolff et al. 2012; Estes et al. 2015 for additional background and information on sample outcomes). This sample allows investigation of brain–behavior relationships across a broad continuum relevant to both typical and atypical developmental outcomes. Participants were enrolled at the following sites: the University of North Carolina, the University of Washington, Children’s Hospital of Philadelphia, and Washington University in St. Louis; the Montreal Neurological Institute serves as the data coordination center. Analyzed participants (n = 187) had both gross motor assessments and neuroimaging data at 12 and/or 24 months (Table 1), ages associated with measurable variation in walking abilities. Exclusion criteria included comorbid medical or neurological diagnoses influencing growth, development, or cognition; known genetic conditions; gestational age < 36 weeks or birth weight < 2000 g; maternal substance abuse during pregnancy; contraindication for MRI; and a first degree relative with psychosis, schizophrenia, or bipolar disorder (Wolff et al. 2012). A clinical best estimate procedure (Estes et al. 2015) determined whether criteria for ASD (autistic disorder or pervasive developmental disorder NOS) were met using the DSM-IV-TR checklist at 24 months (American Psychiatric Association 2000). Children in the low-risk group who obtained ASD diagnoses were removed from analysis, as a goal of IBIS is to examine ASD-related factors in the context of high familial risk. Informed consent approved by each site’s Human Subjects Review Board was obtained for all families.
Table 1.
Participant characteristics
| 12-Month age group (n = 130) | 24-Month age group (n = 99) | |
|---|---|---|
| Age in months | 12.4 (0.4) | 24.5 (0.5) |
| [11.7–14.5] | [23.5–25.9] | |
| Sex [n (% males)] | 86 (66.2%) | 56 (56.6%) |
| Outcome group (%) | ||
| Low-risk negative (no ASD) | 37 (30.8) | 23 (23.5) |
| High-risk negative (no ASD) | 72 (60.0) | 59 (60.2) |
| High-risk positive (has ASD) | 11 (9.2) | 16 (16.3) |
| Site | ||
| CHOP | 18 | 5 |
| UW | 23 | 18 |
| WUSTL | 71 | 53 |
| UNC | 18 | 23 |
| Mullen early learning composite | 99.5 (13.7) | 99.0 (20.5) |
| [64–132] | [49–137] | |
| Mullen raw gross motor score | 16.3 (2.5) | 26.1 (2.5) |
| [9–22] | [18–31] | |
| Mullen walking item score | 1.4 (0.9) | 4.7 (1.1) |
| [0–3] | [2–7] | |
Characteristics of 12 and 24-month age groups are shown for subjects with motor and neuroimaging data. Forty-two subjects had data at both ages, 88 subjects had data at 12 months only, and 57 subjects had data at 24 months only. Standard deviations are in parentheses except where indicated. Ranges are in brackets. Negative and positive refer to absence or presence of an ASD diagnosis. CHOP refers to Children’s Hospital of Pennsylvania. UW refers to University of Washington. WUSTL refers to Washington University in St. Louis. UNC refers to University of North Carolina.
Mullen Scales of Early Learning
The Mullen (Mullen 1995) is a direct behavioral assessment of cognitive development based on demonstration of developmental milestones. Raw scores used in analyses involved 2 sets of items: (1) a “walking score,” comprised of items describing walking-related behaviors (gross motor items 14, 16, 20, 24, 25, 28, 29, 30) and (2) the Mullen gross motor subscale, a standardized index of general gross motor development including walking items as well as items pertaining to postural control, sitting, and running. The gross motor scale is validated up to 33 months of age in typically developing populations and has shown convergent validity with other developmental motor scales (Shank 2011).
Imaging Acquisition
Infants were scanned during natural sleep on identical, cross-site calibrated 3-T Siemens TIM Trio scanners (Siemens Medical Solutions, Malvern, PA) equipped with standard 12-channel head coils. Neuroimaging sequences included T1-weighted and T2-weighted anatomical imaging and fcMRI. This study used the 3-D sagittal T2-weighted sequence for coregistration with the BOLD scan (TE = 497 ms, TR = 3200 ms, matrix 256 × 256 × 160, 1mm3 voxels). fcMRI functional images were collected as a gradient-echo echo planar image (EPI), sensitive to changes in T2* BOLD signal (time echo [TE] = 27 ms, time repetition [TR] = 2500 ms, voxel size 4 × 4 × 4 mm3, flip angle 90°, field of view 256 mm, matrix 64 × 64, bandwidth 1906 Hz). Cross-site fcMRI quality control (QC) was performed (Pruett et al. 2015). All analyzed infants provided at least 2 fMRI runs, each run comprising 130 temporally contiguous frames (6.25 min).
fMRI Preprocessing
Data were preprocessed to reduce artifacts using previously described procedures (Smyser et al. 2010), including sinc interpolation to compensate for slice-dependent time shifts, correction of systematic odd–even slice intensity differences from interleaved acquisition, spatial realignment to compensate for head motion, and within-run intensity normalization to a whole brain mode value of 1000 (Ojemann et al. 1997).
The fMRI data for each participant were spatially registered to an atlas using a sequence of affine transformations. To account for morphological changes across development (Fonov et al. 2011), this affine transformation was calculated by combining a transform from the subject-specific space to age-specific atlas-representative targets (constructed from IBIS subjects) and the transform from the age-specific targets to the final atlas target used at the Washington University School of Medicine’s Neuroimaging Laboratory (Smyser et al. 2010). After fMRI to T2-weighted atlas transform composition, the volumetric time series were resampled in atlas space (3 mm × 3 mm voxels). Finally, to exclude any additional errors, each atlas-transformed functional dataset was visually inspected in sagittal, transverse, and coronal views.
Definition of Regions of “Noninterest” in Atlas Space
White matter and CSF regions were manually defined in atlas-transformed T1-weighted images, which represented 15 subjects in each age group (Pruett et al. 2015). To reduce the risk of intruding on gray matter, regions were eroded using a 2.5 mm Gaussian blurring kernel. The intersection over each age group was computed to create white matter and CSF regions.
Frame Censoring
A rigorous motion-correction algorithm based on the frame-to-frame displacement measure, which records head movement from one volume to the next, was applied to eliminate BOLD frames with frame displacement (FD) > 0.2 mm. The FD measure was calculated as the sum of the absolute values of the 6 different realignment estimates (X ,Y, Z, pitch, yaw, roll) at every time point (Power et al. 2014). Temporally isolated (fewer than 6 contiguous) FD < 0.2 mm frames were also censored and runs with <30 uncensored frames were discarded. To control for potential biases attributable to the amount of data per cohort, 150 noncensored (retained) fMRI frames were used, prioritizing runs with the most retained frames, for correlation analysis in each subject. In total, 82% of participants with usable scans passed QC for scrubbing at 12 months and 81% passed QC for scrubbing at 24 months. Rates of scans passing QC did not differ by high and low-risk outcome groups (12 months: χ2(2) = 1.89, P = 0.39; 24 months: χ2(2) = 4.59, P = 0.10).
fcMRI Preprocessing
Data were demeaned and detrended at every voxel within runs, minus censored frames (Power et al. 2014). To mitigate artifacts related to motion, which confound interpretations of fc, particularly in studies of development where age is a factor of interest (Power et al. 2012; Satterthwaite et al. 2013; Yan et al. 2013; Tyszka et al. 2014), nuisance waveforms were regressed voxelwise. These included time series derived from three translation (X, Y, Z) and three rotation (pitch, yaw, roll) estimates derived by retrospective head motion correction, as well as Volterra expansion derivatives (24 total motion regressors) (Friston et al. 1996) and time series derived from the whole brain, white matter, and cerebrospinal fluid and their first derivatives. Data in censored frames were replaced by interpolated values computed by least-squares spectral analysis (Mathias et al. 2004; Power et al. 2014). Interpolated data were only included for bandpass filtering and did not factor into time-series correlations. The data were temporally filtered to retain frequencies in the 0.009 Hz < f < 0.08 Hz band and then spatially smoothed using a 6 mm full-width at half maximum (FWHM) isotropic Gaussian kernel.
Definition of ROIs and Correlation Computation
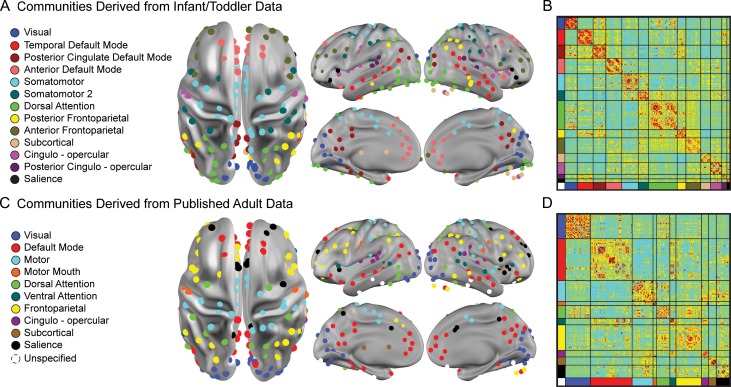
Regions of interest (ROIs) were selected based on 280 ROIs pooled from (1) meta-analyses of task data and cortical functional areal parcellations obtained in healthy adults (Cohen et al. 2008; Power et al. 2011) and (2) a meta-analysis of autism studies (Philip et al. 2012). These well-validated, functionally defined ROIs, which reflect the underlying functional organization of the brain (Power et al., 2011), were identified in individuals with and without ASD, consistent with the current sample, and were selected to enhance the neurobiological validity and generalizability of findings relative to well-vetted task-based data from the literature. Fifty ROIs were removed for lying partially outside the whole brain mask or for showing inconsistent gray matter coverage across age. The final 230 usable ROIs (Fig. 1) included 218 from Power et al. (2011) and 12 from Phillip et al. (2012), which have been applied in three previously published analyses of IBIS data (Pruett et al. 2015; Eggebrecht et al. 2017; Emerson et al., 2017). ROI time series were calculated as the average of the time series of each voxel contained within the 10 mm-diameter sphere at a given ROI center. Fc values were calculated as the pairwise zero-lag Pearson correlations for each of the 26 335 ROI pairs and then Fisher-z transformed to improve normality.
Figure 1.
Infomap-derived network models based on infant/toddler and adult data. (a) Putative infant–toddler networks were derived from participants with fcMRI data at 12 and 24 months (n = 48). The 230 functionally defined ROIs comprising these networks are colored by network assignment. Naming of networks was informed by previously published adult networks. (b) A mean fcMRI adjacency matrix for 12-month data displays the 230 ROIs sorted by network. (c) An adaptation of previously published adult networks (Power et al. 2011) is based on the same 230 ROIs. (d) A mean 12-month fcMRI adjacency matrix using the adult networks is shown.
Derivation of Putative Functional Networks
To create an infant/toddler network model (Fig. 1a), the complete set of ROI-pair fc correlations (Fisher-z values) for 48 IBIS subjects with fcMRI data at both 12- and 24-month visits (including subjects without motor scores) were averaged across subjects, producing a 230 × 230 connection matrix (nodes = ROIs, edges=correlation coefficients). The averaged set of correlations was thresholded and binarized at multiple thresholds to generate connection matrices with sparseness ranging from 1% to 10% of all possible surviving connections at steps of 0.1%, yielding 91 total edge density thresholds. Connections between ROI pairs separated by <20 mm were removed to minimize the effects of blurring in the fMRI data. The Infomap community detection algorithm (Rosvall and Bergstrom 2008), which assigns ROIs to communities of putative networks based on maximization of within-module random walks in the connection matrix, was then applied to connection matrices at each threshold. Communities with ≤5 ROIs were labeled “Unassigned” and removed. Solutions for each threshold were combined using an automated “consensus” procedure to provide a single model of the community structure by maximizing the normalized mutual information of groups of neighboring solutions and then maximizing modularity (Eggebrecht et al. 2017). This cross-age, infant–toddler network solution (Fig. 1), which included infants at high and low risk of ASD, provided a network model to facilitate enrichment analyses for each age group as well as comparison of brain–behavior correlations across 12 and 24 months (see below). As adult brain networks have been more extensively characterized than infant networks, an additional network structure applying the same 230 ROIs was generated from a previously published fcMRI dataset of typical adults (Power et al. 2011) to provide a secondary comparison (Fig. 1b).
Statistical Analysis
Enrichment Analysis
Analyses were conducted in Matlab 15. We closely followed the procedure recently published by Eggebrecht et al. (2017). Enrichment analysis is an established data-driven statistical method from genome-wide association studies (Rivals et al. 2007; Backes et al. 2014; Khatri et al. 2012). It detects differential involvement of factors related to an outcome of interest, while constraining the burden of multiple comparisons, a frequent challenge for brain-wide neuroimaging analyses. We chose a brain-wide approach because of the limited infant literature on brain–behavior relationships for motor function. We applied enrichment analysis to identify networks with a significantly increased density of connections (i.e., between pairs of ROIs either within or across networks), whose fc strongly correlated with motor behavior. Results are reported in terms of significantly enriched network blocks, which consist of either a single network or pair of brain networks, thereby describing intranetwork and internetwork fc-motor relationships.
Separate enrichment analyses were performed for walking, the primary analysis, which involved a specific behavior, and for gross motor behavior, the secondary analysis, which provided a greater dynamic range of scores representing multiple aspects of gross motor function. Spearman correlations were used for fc-motor correlations given the non-normal distribution of motor scores. Brain–behavior correlation values for ROI pairs were thresholded and binarized at an uncorrected P-value ≤ 0.05. Two complementary test statistics, a 1-degree of freedom χ2 test and a hypergeometric statistic, were used to test for enrichment of network blocks (see Eggebrecht et al. 2017 for further details). Network blocks considered enriched were required to be significant on both tests, as a conservative threshold. The McNemar χ2 statistic was used to test for significant differences in network enrichment between 12 and 24-month age groups.
As a second stage of analysis, permutation testing was conducted to determine empirical significance level for results from the χ2, hypergeometric, and McNemar tests (Eggebrecht et al. 2017). This empirical significance level represents the 5% false positive rate for enrichment. In permutation testing, the fcMRI-motor data pairing, which preserved fc correlations and missing-data patterns, was randomized for each of 100 000 iterations. At each iteration, permutations of complete fcMRI matrices were conducted separately on subjects with data at 12-month only, 24-month only, or repeated measures at both time points. Longitudinal subjects were then recombined with cross-sectional subjects to produce 2 separate brain-wide null distributions—one per time point. The permutation-based false-positive-rate did not exhibit any correlation with the number of ROIs within a network pair (Walking 12 months: r = 0.0050, P = 0.96; Walking 24 months: r = −0.12, P = 0.25; Gross Motor 12 months: r = −0.031, P = 0.77, Gross Motor 24 months: r = −0.15, P = 0.17), confirming that the size of the network pair does not bias results.
To qualify as significant in the enrichment analysis, an enriched network block had to show either enrichment at both time points, or enrichment at one time point plus a significant difference from a null finding of no enrichment at the other time point. Network blocks enriched at one time point only and not significantly different from the other time point represent discovery results for future hypothesis generation.
Neurosynth Review
The online Neurosynth platform (www.neurosynth.org) (Yarkoni et al. 2011) contains a database of activation coordinates from over 11 406 published studies (as of 16 September 2016). The database provides a metric for the likelihood of the association between functional activation at an ROI’s coordinates and prespecified search terms. Publications reporting activation at an ROI can also be searched for the high-frequency use of specific behavioral terms of interest.
Searches for frequently connected ROIs, specified as the top 25% of ROIs contributing to enriched network blocks, were conducted in Neurosynth to systematically explore whether these ROIs were implicated in studies of motor function. MNI coordinates for these highly connected ROIs were entered into Neurosynth to determine the likelihood of association with the search term “motor,” which also referenced words or phrases containing “motor,” for example, “sensorimotor” and “motor performance.” The standard search setting of 6 mm was used to identify functional activations for each ROI. For each motor-related term, Neurosynth provided a posterior probability value indicating the likelihood of that term being frequently used in a manuscript reporting activation at a given ROI (Yarkoni et al. 2011). Posterior probabilities for general motor terms (e.g., “sensorimotor”) and terms indicating a motor behavior (e.g., “motor response”) were ranked separately. For these 2 categories, terms with the top 3 posterior probabilities >0.5 were reported for each ROI (Table S2). Next, publications containing a functional activation for a given ROI were searched for whether they included the following motor-related terms: motor, walk, gait, or biological motion. Each identified study was reviewed to confirm the relevance of the functional activation at that ROI for motor behavior.
Results
Participant Characteristics and Motor Score Distributions
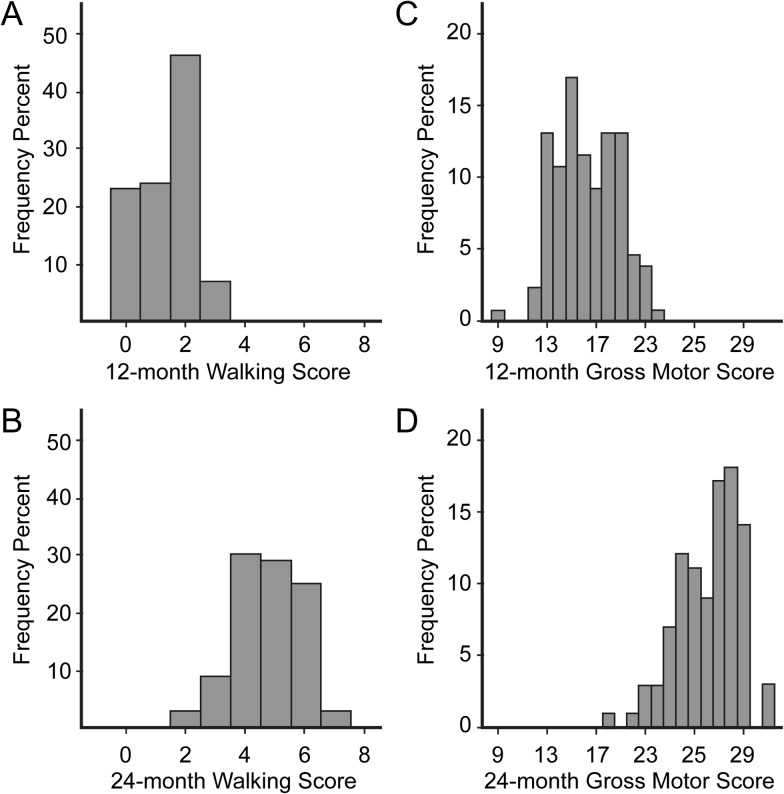
Participant characteristics for this sample of LR and HR infants (n = 130 at 12 months, n = 99 at 24 months, with n = 42 having data at both time points) are shown in Table 1 (see supplement for analyses comparing characteristics at each time point). The distribution of walking item scores at 12 months showed low values, consistent with emergence of this skill (Fig. 2), while the distribution at 24 months showed higher scores (t[227] = −25.01, P < 0.0001), consistent with developmental progress. Raw gross motor scores, which index several gross motor behaviors and include walking items, demonstrated a continuous, unimodal distribution at both time points (Fig. 2) and higher scores at 24 months (t[227] = −29.39, P < 0.0001).
Figure 2.
Walking and gross motor scores are dimensionally distributed at 12 and 24 months. Distributions of raw scores are shown for subjects with brain and behavioral data at 12 (n = 130) and 24 months (n = 99). (a) 12-month walking scores display a range of low values, consistent with emergence of walking at this age. (b) Greater walking scores at 24 months confirm developmental progress. (c) 12-month gross motor scores represent behaviors in addition to walking and show a broader continuous distribution. (d) 24-month gross motor scores are greater than 12-month scores.
Enrichment Analysis: Walking Scores at 12 and 24 Months
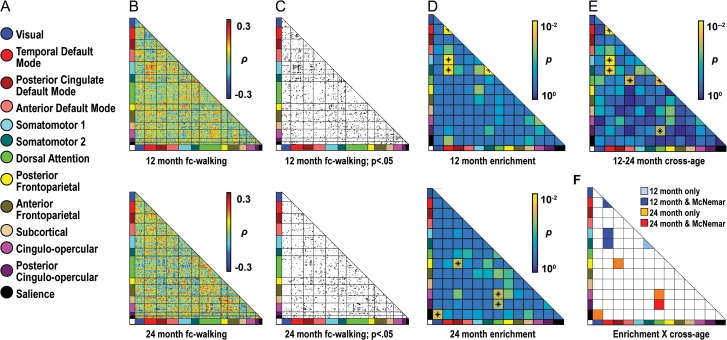
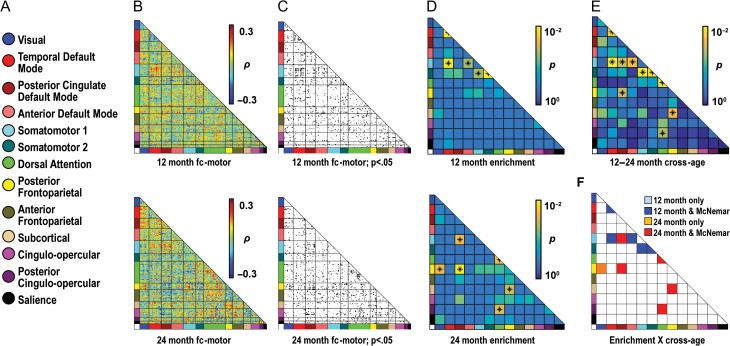
At 12 months, 4 network blocks were enriched (Fig. 3, Table S1 for enrichment statistics): tDMN–SMN, temporal default mode–somatomotor 2 (tDMN–SMN2), tDMN (tDMN–tDMN), and SMN 2 (SMN2–SMN2). At 24 months, 4 nonoverlapping network blocks were enriched, which implicated a greater variety of networks than at 12 months: dorsal attention–posterior cingulo-opercular (DAN–pCO), DAN–cingulo-opercular (DAN–CO), visual-salience (Vis-Sal), and posterior cingulate default mode–posterior frontoparietal (pcDMN–pFPC).
Figure 3.
Enrichment analyses show specific brain–behavior relationships for walking scores at 12 and 24 months. (a) A color-coded key of 13 putative infant/toddler networks is shown. (b) Matrices describe relationships between functional connectivity (fc) and walking score. Data at 12 and 24 months are shown in the top and bottom rows, respectively (b–d). The 230 ROIs comprising these networks are sorted by assigned network along the X and Y axes. Hot colors indicate strong positive relationships of fc to walking score; whereas cool colors indicate strong negative fc-walking relationships. (c) The fc-walking matrices for 12 and 24 months are thresholded to show ROI pairs with fc-walking correlations significant at an uncorrected threshold of P ≤ 0.05. (d) Matrices are colored by P-values for enrichment. Enriched blocks are labeled with an asterisk. For simplicity, results for χ2-square testing, which are comparable to the hypergeometric test, are shown in all figures. (e) This matrix is colored by P-values for the McNemar test, which evaluated differences in the level of enrichment at 12 versus 24 months. Asterisks indicate significant differences. (f) Blocks are colored based on whether they are enriched at a given time point and whether their level of enrichment also differs between time points. Here, significant network blocks (dark blue and red squares) are enriched at either 12 or 24 months and significantly different between 12 and 24 months. Other findings are discovery results.
The nonoverlapping findings at 12 and 24 months suggest that network profiles of enrichment differ across age. Significant differences in levels of enrichment were observed at 12 versus 24 months (Fig. 3e) and included network blocks also enriched at either 12 or 24 months (Fig. 3f). Three of the 4 network blocks identified at 12 months, tDMN–tDMN, tDMN–SMN, and tDMN–SMN2, also showed differences in enrichment compared with 24 months and therefore constituted significant findings. Only one network block found at 24 months, DAN–pCO, differed significantly from 12 months and qualified as significantly enriched. Network blocks enriched at a single age without a significant difference across time points (e.g., SMN2–SMN2), are presented in figures as discovery results.
Enrichment analyses using Infomap-derived communities from previously published adult data (Fig. 1b) identified 2 network pairs at 12 months which also differed in levels of enrichment from 12 to 24 months (and were therefore significant findings): motor–DMN and motor–ventral attention (Fig. S1; enrichment statistics in Table S1). At 24 months, a single enriched network pair, salience–frontoparietal, similarly qualified as a significant finding.
fc-Walking Relationships Within and Across Networks
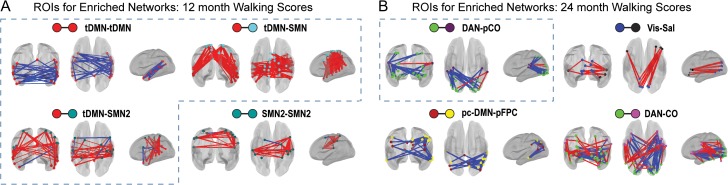
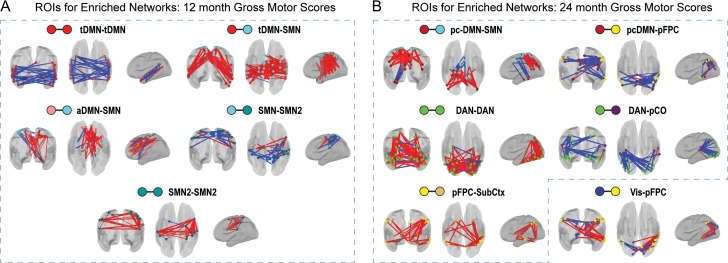
We next examined whether correlations between fc and walking score were positive or negative in enriched network blocks. Among significant network blocks at 12 months, tDMN–tDMN showed uniformly negative brain behavior relationships (blue lines), whereas tDMN–SMN, and tDMN–SMN2 showed predominantly positive brain–behavior relationships (red lines) (Fig. 4). Hence, within the tDMN, lower fc values were associated with higher walking scores, whereas for tDMN–SMN and tDMN–SMN2, greater fc values were associated with higher walking scores. At 24 months, DAN–pCO, the one significant network block, exhibited ROI pairs with largely negative fc-walking relationships (Fig. 4).
Figure 4.
The sign of fc-walking relationships is generally consistent within enriched network blocks. Locations and signs of brain–behavior relationships are illustrated for ROI pairs contributing to enrichment. Spheres represent ROIs. Red and blue sticks represent positive or negative brain–behavior relationships, respectively, between functional connectivity (fc) for an ROI pair and walking scores. (a) At 12 months, all enriched network blocks show primarily positive or negative fc-walking relationships. (b) Similar uniformity occurs at 24 months, with the exception of DAN–CO. Hatched boxes enclose significant versus discovery results.
We additionally evaluated whether ROI pairs contributing to enriched network blocks exhibited predominantly positive or negative fc values, reflecting a history of coactivation or anti-correlated activity, respectively. Both positive and negative fc values were observed at ROI pairs contributing to enriched networks (Supplemental results, Fig. S2).
Enrichment Analysis: Gross Motor Scores at 12 and 24 Months
As a secondary analysis, fc-behavior relationships were examined for raw scores on the Mullen gross motor subscale, permitting comparison of brain–behavior findings for walking to a more comprehensive representation of gross motor ability. Because walking items contributed to gross motor scores, we anticipated some similarity in enriched networks but not complete overlap.
At 12 months, 5 network blocks were enriched and constituted significant findings: tDMN (tDMN–DMN), tDMN–SMN, anterior default mode–SMN, SMN–SMN2, and SMN2–SMN2 (Fig. 5). Three of these 5 blocks, tDMN–tDMN, tDMN–SMN2, and SMN2-SMN2, were also identified in analyses for walking. None of these blocks were enriched at 24 months, and all showed significantly different levels of enrichment between 12 and 24 months.
Figure 5.
Enrichment analyses show specific brain–behavior relationships for gross motor scores at 12 and 24 months. (a) A color-coded key of 13 putative infant/toddler networks is shown. (b) Matrices describe relationships between functional connectivity (fc) and gross motor score. Data at 12 and 24 months are shown in the top and bottom rows, respectively (b–d). The 230 ROIs comprising the networks are sorted by assigned network along the X and Y axes. Hot colors indicate strong positive relationships of fc to gross motor score; whereas cool colors indicate strong negative fc-gross motor relationships. (c) The fc-gross motor matrices for 12 and 24 months are thresholded to show ROI pairs with fc-gross motor correlations significant at an uncorrected threshold of P ≤ 0.05. (d) Matrices are colored by P-values for enrichment. Enriched blocks are labeled with an asterisk. (e) This matrix is colored by P-values for the McNemar test, which evaluated differences in the level of enrichment at 12 versus 24 months. Asterisks indicate significant differences. (f) Blocks are colored based on whether they are enriched at a given time point and whether their level of enrichment also differs between time points. Here, significant network blocks (dark blue and red squares) are enriched at either 12 or 24 months and significantly different between 12 and 24 months.
At 24 months, 5 network blocks qualified as significantly enriched: DAN–pCO, which was also significant for walking at 24 months, DAN–DAN, pcDMN–SMN, pcDMN–pFPC, and posterior frontoparietal–subcortical (pFPC–SubCtx) (Fig. 5). All blocks showed differences in enrichment between 12 and 24 months. Similar to findings for walking, no network blocks overlapped at 12 and 24 months, although the SMN was part of enriched network blocks at 12 and 24 months. Increased involvement of attentional and task control networks was observed at 24 months, as seen in fc-walking analyses. In contrast to infant–toddler networks, enrichment analyses using adult networks showed no significant findings (Fig. S4).
Fc-Gross Motor Relationships Within and Across Networks
Similar to walking results, positive and negative fc-gross motor relationships were found at 12 and 24 months, and the sign of fc-gross motor relationships for ROI pairs contributing to enrichment was generally consistent within network blocks (Fig. 6). The predominant sign of these relationships was the same for all significant network blocks from both walking and gross motor analyses (i.e., tDMN–tDMN, tDMN–SMN at 12 months, and DAN–pCO at 24 months). In blocks involving the SMN, both positive fc-gross motor relationships (i.e., aDMN–SMN, tDMN–SMN, SMN2–SMN2, and pcDMN–SMN) and a negative fc-gross motor relationship (SMN–SMN2) were observed. At 24 months, a positive fc-gross motor relationship was noted for pFPC–SubCtx, which included SubCtx ROIs located in the basal ganglia and cerebellum, regions associated with motor function. Information on the sign of fc for ROI pairs contributing to enrichment is available in supplemental material (Figs S5 and S6).
Figure 6.
The sign of fc-gross motor relationships is generally consistent in enriched network blocks. Locations and signs of brain–behavior relationships are illustrated for ROI pairs contributing to enrichment. Spheres represent ROIs. Red and blue sticks represent positive or negative brain–behavior relationships, respectively, between functional connectivity (fc) for an ROI pair and gross motor scores. At 12 months (a) and 24 months (b), all network blocks show primarily positive or negative relationships between fc and gross motor scores. Hatched boxes enclose significant versus discovery results.
ROI-Level Associations With Motor Behavior
Because enrichment analyses implicated motor networks as well as networks less directly associated with motor behavior, we investigated whether ROIs contributing to enriched network blocks for walking, the primary behavior analyzed, were associated with motor function according to prior literature. To systematize our literature search, we selected those ROIs demonstrating a high frequency of connections in significantly enriched network blocks (Materials and Methods) and reviewed these ROIs in Neurosynth (Yarkoni et al. 2011), a database containing information on whether functional activations of these ROIs is associated with motor-related behaviors. Our search confirmed associations with several motor-related behaviors, including motor execution (Fink et al. 1997; Zapparoli et al. 2013; Gandolla et al. 2014), action observation (Iseki et al. 2008; Wagner et al. 2008; Villiger et al. 2013), visuomotor integration (Iacoboni and Zaidel 2004; Martuzzi et al. 2006), biological motion detection (Vaina et al. 2001; Bidet-Caulet et al. 2005), motor learning (Sacco et al. 2009; Lungu et al. 2014), motor inhibition (Nakata et al. 2008, Cai et al. 2014), and motor imagery (Cremers et al. 2012; van der Meulen et al. 2014; Taube et al. 2015) (Table S2). Further, ROIs from several networks were associated with behaviors relevant to walking, including motor execution involving the leg or ankle, motor imagery related to gait, and biological motion detection of walking.
Discussion
Here we applied a data-driven approach in a large sample to provide an initial description of brain–behavior relationships for network-level fc and walking and gross motor function at ages 12 and 24 months, a period of rapid development. Our findings indicate that (1) subsets of infant/toddler networks show strong relationships of fc to walking and gross motor scores, (2) the profile of these networks differs at 12 and 24 months, (3) these network profiles involve both positive and negative brain–behavior relationships, and (4) highly connected ROIs from networks associated with walking have been implicated in the execution and regulation of motor activities in the adult literature. Network profiles were similar but not identical for walking and gross motor function, suggesting the enrichment analysis detected differences related to unique variance from these behavioral measures. Recently published work in an overlapping sample from IBIS also supports the specificity of our findings, as a distinct profile of enriched networks was related to initiation of joint attention, a pivotal feature of social development (Eggebrecht et al. 2017).
Age-related differences in enriched networks suggest that network substrates of these behaviors are dynamic in early life, while the observed positive and negative brain–behavior relationships additionally imply that increases and decreases in network-level connectivity may underlie the characteristic developmental progression of these behaviors. Interestingly, although network-level brain–behavior relationships differed at ages 12 and 24 months, highly connected ROIs in enriched networks were associated with motor function in adults. Thus, brain–behavior relationships at distinct levels of neural architecture, in this case networks and ROIs, may provide a mechanism for both change and continuity in neural substrates of behavior during development.
Results of Enrichment Analysis Demonstrate Face Validity
Because the somatomotor networks derived for this study span known areas of somatosensory and motor cortex (S1, M1, and other regions in the precentral and postcentral-gyri—Fig. 1), we could evaluate the face validity of findings from enrichment analysis, an approach recently adapted for neuroimaging data (Eggebrecht et al. 2017). Consistent with our hypothesis, motor network involvement was observed for walking and gross motor scores at 12 months and gross motor scores at 24 months. Further, the Neurosynth review demonstrated that highly connected ROIs in enriched network blocks from walking analyses have been implicated in motor function. These results thus support the utility of enrichment analysis both for the identification of network-level brain–behavior relationships, and as a data reduction approach to identify ROI-level brain–behavior relationships, which would not have otherwise survived corrections for multiple comparisons.
Enrichment analyses using adult networks for walking scores confirmed motor network involvement while demonstrating a gross degree of somatotopic specificity, since the “motor mouth” network, which lacks lower limb ROIs, was not enriched. Use of adult networks in secondary analyses of gross motor scores, however, failed to detect any significant brain–behavior relationships. Given the strong face validity of findings with the infant/toddler networks, this discrepancy highlights the importance of using developmentally specific networks to enhance the sensitivity of the enrichment analysis.
Infant/Toddler Findings Parallel and Extend Existing Motor Literature
Work in both human and nonhuman primates has shown that brain bases of motor behavior include primary motor areas, such as primary motor cortex, and more broadly distributed nonprimary motor areas, including somatosensory cortex, supplementary motor areas, premotor cortex, dorsal cingulate regions, and parietal regions, as well as the cerebellum (Fink et al. 1997; Rizzolatti and Luppino 2001; Hanakawa et al. 2003). These regions contribute directly to the execution of motor activities and to aspects of perception and cognition important for motor function. Walking and gross motor findings were both consistent with the prevailing literature, in that they revealed involvement of networks encompassing primary and nonprimary motor areas. Examination of highly connected ROIs in significantly enriched networks for walking confirmed an association with motor execution, including gait and foot movement, as well as motor inhibition and motor learning. The striking convergence of motor associations in adults for ROIs identified in our infant/toddler analyses implies continuity at the level of individual ROIs for fc-motor relationships originating early in development.
The motor network relationships involving the DMN, observed in both walking and gross motor analyses, were unexpected. At 12 months, intranetwork fc within the tDMN was negatively associated with walking and gross motor function, whereas internetwork fc between the tDMN and motor networks was positively associated with walking and gross motor function. Thus, the tDMN demonstrated a pattern of inverse intranetwork and internetwork fc-motor relationships, whereby increases in fc within the tDMN were associated with less advanced motor function, while increases in fc between the tDMN and motor networks were associated with more advanced motor function. These inverse relationships operated at the level of specific tDMN ROIs which contributed to both intranetwork enrichment in the DMN and cross-network enrichment of tDMN and SMN and SMN2 (see Supplemental Results).
Associations of the DMN with motor function are infrequent in the literature, in contrast to the DMN’s postulated role in self-referential processing (Raichle 2015). Interestingly, ecological theories of perceptual development have proposed an interrelationship between early gross motor development and self-perception, by positing that gross motor skills, including walking, enhance children’s ability to obtain information from the environment and to perceive themselves in the process (Gibson and Pick 2000). Our finding of frequent associations for highly connected tDMN ROIs and motor imagery further support an intersection of motor abilities and self-perception, as motor imagery requires imagining oneself performing motor actions. Thus, the strong findings for the DMN may reflect a developmental link between acquisition of early gross motor skills and aspects of self-referential processing.
Networks Linked to Higher-order Cognition Play a Role in Later Gross Motor Development
SMN and tDMN were strongly implicated in walking and gross motor function at 12 months, but not at 24 months. Rather, networks associated with higher-order cognition, namely DAN and pCO, a task control network, exhibited cross-network connectivity related to both walking and gross motor function, with fc of another task control network, the pFPC network, being related specifically to gross motor function. This qualitative difference corresponds to dramatic developmental advances encompassing the emergence, refinement, and elaboration of walking and gross motor skills. Cognitive processes mediated by the DAN and task control networks, including orientation towards external stimuli (Corbetta and Shulman 2002) and regulation of goal-directed behavior (Dosenbach et al. 2007), respectively, may therefore be important for the progression of walking and gross motor abilities.
The cross-age shift to networks linked to higher-order cognitive processes also parallels other investigators’ findings about developmental courses of the networks, themselves. Prior work in infants has shown that motor networks mature initially, followed by networks mediating higher-order cognitive functions in adults (Gao et al. 2015b). This maturational difference additionally corresponds to differences in network-level properties in adults. The DMN and motor network, both enriched at 12 months, have previously been described in adults as “processing” networks, which are locally well-integrated and relatively isolated in relation to other functional systems (Power et al. 2011). In contrast, the FPC network, enriched in the 24-month gross motor results, displayed less local integration and greater participation with other functional systems in adults (Power et al. 2011). Earlier acquisition of gross motor skills is thus associated with fc of networks which are ultimately more self-integrated, whereas elaboration of these skills involves networks whose architecture ultimately favors cross-network integration, a characteristic thought to promote the accommodation of a wider range of tasks.
Lastly, differences in the sign of fc-gross motor relationships for the tDMN and DAN beg consideration of previously described differences in the roles of these networks. At 12 months, the tDMN displayed a negative intranetwork brain–behavior relationship, whereas at 24 months, the intranetwork brain–behavior relationship for DAN was positive. In adults, an anti-correlated relationship for the DMN and DAN has been reported during specific task-based activities, with DMN belonging to the “task negative” system (Raichle et al. 2001; Lin et al. 2011) and DAN being associated with the “task positive” system (Fox et al. 2005; Power et al. 2011). Anti-correlated resting state fc has also been demonstrated for these networks (Corbetta and Shulman 2002), and recent evidence supports the emergence of these resting state anticorrelations across age in infants (Gao et al. 2013). Our findings raise the question of whether inverse functional relationships for the DMN and DAN, which occur during task-related activation and at rest, may also support early gross motor development.
fc-Motor Relationships Promote Insight Into ASD
Gross motor delays in infancy have been associated with later ASD diagnosis (Lloyd et al. 2013; Estes et al. 2015), as well as deficits in early social communication (LeBarton and Iverson 2016), implying that neural systems supporting gross motor development could contribute to social impairment in ASD, which generally manifests in toddlerhood. Consistent with this notion, several networks identified in the enrichment analyses, including the SMN, DMN, and DAN, have shown altered connectivity in ASD (Mostofsky et al. 2009; Fitzgerald et al. 2015; Jann et al. 2015; Abbott et al. 2016), and connectivity of the DMN (Yerys et al. 2015) and motor network (Nebel et al. 2014a, 2016) have also been correlated with core social symptoms. The basal ganglia, a known regulator of motor execution, was identified via the subcortical network in fc-gross motor analyses at 24 months and has been implicated in altered social reward processing in ASD (Scott-Van Zeeland et al. 2010). Highly connected ROIs in our study were also related, per the literature, to cognitive processes disrupted in ASD, including visuomotor learning, a motor deficit specific to ASD (Nebel et al. 2016), as well as biological motion detection, action observation, and imitation, which have been hypothesized to promote social communication. The identification of these associations by age 12 months suggests that neural networks underlying gross motor development may contribute to the emergence of ASD. Infant brain-behavioral studies of motor development could thus illuminate neural markers of ASD risk and inform future motor-based, early interventions targeting experience-dependent development of vulnerable neural networks in ASD.
Limitations
Enrichment analysis identified networks most strongly related to walking and gross motor behavior, and complementary methods could reveal additional fc-motor relationships not detected with this method. Network solutions were based on functionally defined ROIs from older subjects rather than infants (as were findings from Neurosynth) due to limited task-based fMRI data in infants. A compelling future direction involves extending enrichment analyses to developmentally specific ROIs using infant and toddler functional areal parcellations, once such techniques have been realized.
Our fMRI processing included global signal regression, an established approach to reliably remove motion-related artifacts from fMRI data (Yan et al. 2013; Power et al. 2014). Recent work involving rigorous head-to-head comparisons in 14 models with and without GSR (Ciric et al. 2017) has demonstrated that GSR in combination with volume censoring (a technique also used in this study) is superior for removing motion-related artifact and that other denoising procedures are relatively ineffective at removing motion-related artifact. However, one limitation of GSR is that current processing techniques cannot also rule out the concomitant removal of genuine neural signal. Because motion-related artifacts present a major confound in fc analyses, particularly when age is a variable of interest (Power et al. 2012; Satterthwaite et al. 2013; Yan et al. 2013; Tyszka et al. 2014), we elected to conservatively account for motion-related artifacts to minimize the risk of spurious interpretations of fc-behavior relationships. Future development of more sophisticated processing methods capable of distinguishing artifacts from genuine global neural signal, as well as better tools to deal with motion during data collection, will be important steps in advancing the understanding of functional brain–behavior relationships.
By including participants with longitudinal and cross-sectional data, these analyses maximized power for the purposes of scientific discovery at each time-point, but cross-age comparisons involved some nonoverlapping subjects. Replication is therefore warranted to test for generalization of our findings to a large, purely longitudinal sample, as well as a large low-risk sample, since brain–behavior relationships may differ in children at high versus low risk for ASD. The current sample, while relatively large, contained few children with ASD (Table 1) and was thus underpowered to test for potential group differences in brain–behavior relationships. Finally, fcMRI data were collected during natural sleep, which may influence underlying fc versus the awake state; these results are nevertheless informative for describing the history of functional brain activations vis-a-vis behavior.
Future Directions
Exciting future directions include applying this approach to longitudinal populations over broader age ranges to compare brain–behavior trajectories among typical and atypical populations, and groups receiving motor interventions. Evaluating ASD-related group differences in an adequately powered sample at younger ages could reveal a predictive neural signature promoting early identification of children who would benefit from interventions, while analyzing aspects of motor function specifically impaired in ASD (e.g., visuomotor integration) could clarify neural systems contributing to ASD’s emergence. Overlap analyses of enrichment for motor abilities and other behavioral domains (e.g., language and joint attention) could illuminate shared neurodevelopmental mechanisms relevant to both typical and atypical development, leading to novel, high impact motor-based interventions capable of stimulating growth in multiple aspects of adaptive function.
Supplementary Material
Notes
We thank the families and children for their time and participation. We thank Leigh MacIntyre for managing the IBIS database. Conflict of Interest: The authors declare the following conflicts of interests: R.C.M. receives acting, modeling, and speaking fees from Siemens Healthcare. A.C.E. is a founder and a member of the Board of Directors of Biospective Inc. All other authors declare no competing financial interests.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
National Institutes of Health (grant R01 MH093510 to J.R.P), a National Institutes of Health Autism Center of Excellence R01 grant (National Institute of Child Health and Human Development) (#HD055741 to J.P.), Autism Speaks (#6020 to J.P.), the Simons Foundation (#140209 to J.P.), the McDonnell Center for Systems Neuroscience (J.R.P.) and a National Institute of Mental Health K01 MH103594 (A.T.E.). National Institute of Mental Health (K08 MH112891 to N.M.) and the Intellectual and Developmental Disabilities Research Center at Washington University (National Institutes of Health/National Institute of Child Health and Human Development P30 HD062171 to J.N.C.).
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, Muller RA. 2016. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. 26:4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association 2000. Diagnostic statistical manual of mental disorders. 4th ed Washington DC: American Psychiatric Association. [Google Scholar]
- Backes C, Ruhle F, Stoll M, Haas J, Frese K, Franke A, Lieb W, Wichmann HE, Weis T, Kloos W, et al. . 2014. Systematic permutation testing in GWAS pathway analyses: identification of genetic networks in dilated cardiomyopathy and ulcerative colitis. BMC Genomics. 15:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. 2012. Motor “dexterity”?: Evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 22:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A, Voisin J, Bertrand O, Fonlupt P. 2005. Listening to a walking human activates the temporal biological motion area. Neuroimage. 28:132–139. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Suwalsky JT. 2013. Physically developed and exploratory young infants contribute to their own long-term academic achievement. Psychol Sci. 24:1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Cannistraci CJ, Gore JC, Leung HC. 2014. Sensorimotor-independent prefrontal activity during response inhibition. Hum Brain Mapp. 35:2119–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. 2000. Travel broadens the mind. Infancy. 1:149–219. [DOI] [PubMed] [Google Scholar]
- Carper RA, Solders S, Treiber JM, Fishman I, Muller RA. 2015. Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. J Am Acad Child Adolesc Psychiatry. 54:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, et al. . 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. 2008. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- Cremers J, Dessoullieres A, Garraux G. 2012. Hemispheric specialization during mental imagery of brisk walking. Hum Brain Mapp. 33:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. 2000. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 71:44–56. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. . 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, Adams CM, Snyder AZ, Lewis JD, Estes AM, et al. . 2017. Joint attention and brain functional connectivity in infants and toddlers. Cereb Cortex. 27:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, Constantino JN, Shen MD, Swanson MR, Elison JT, et al. . 2017. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 9(393). pii:eaag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, St. John T, Paterson S, Elison JT, Hazlett H, Botteron K, Dager SR, Schultz RT, et al. , IBIS Network . 2015. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. 1997. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 77:2164–2174. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Johnson K, Kehoe E, Bokde AL, Garavan H, Gallagher L, McGrath J. 2015. Disrupted functional connectivity in dorsal and ventral attention networks during attention orienting in autism spectrum disorders. Autism Res. 8:136–152. [DOI] [PubMed] [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. 2012. Head lag in infants at risk for autism: a preliminary study. Am J Occup Ther. 66:577–585. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative Group . 2011. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 54:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. 2010. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 40:1227–1240. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 21:145–154. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med. 35:346–355. [DOI] [PubMed] [Google Scholar]
- Gandolla M, Ferrante S, Molteni F, Guanziroli E, Frattini T, Martegani A, Ferrigno G, Friston K, Pedrocchi A, Ward NS. 2014. Re-thinking the role of motor cortex: context-sensitive motor outputs? Neuroimage. 91:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. 2015. a. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex. 25:2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. 2015. b. Development of human brain cortical network architecture during infancy. Brain Struct Funct. 220:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen S, Smith JK, Zhu H, Lin W. 2013. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 23:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Grewen K, Gilmore JH. 2016. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 23:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Sundaram R, Bell E, Bello SC, Kus C, Yeung E. 2016. Gross motor milestones and subsequent development. Pediatrics. 138:e2015–e4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ, Pick AD. 2000. An ecological approach to perceptual learning and development. New York (NY): Oxford University Press. [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. 2015. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 12:12–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. 2003. Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 89:989–1002. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, McKinstry RC, Shaw DW, Botteron KN, Dager SR, Styner M, Vachet C, Gerig G, Paterson SJ, et al. , IBIS Network . 2012. Brain volume findings in 6-month-old infants at high familial risk for autism. Am J Psychiatry. 169:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. 2001. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. Int J Lang Commun Disord. 36:149–171. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Zaidel E. 2004. Interhemispheric visuo-motor integration in humans: the role of the superior parietal cortex. Neuropsychologia. 42:419–425. [DOI] [PubMed] [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. 2008. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 41:1021–1031. [DOI] [PubMed] [Google Scholar]
- Jann K, Hernandez LM, Beck-Pancer D, McCarron R, Smith RX, Dapretto M, Wang DJ. 2015. Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav. 5:e00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser ML, Schoemaker MM, Albaret JM, Geuze RH. 2014. What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Res Dev Disabil. 36C:338–357. [DOI] [PubMed] [Google Scholar]
- Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Muller RA. 2015. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry. 78:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Sirota M, Butte AJ. 2012. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 8:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarton ES, Iverson JM. 2016. Associations between gross motor and communicative development in at-risk infants. Infant Behav Dev. 44:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G, Moustafa AA, Shafir T. 2016. Thinking, walking, talking: integratory motor and cognitive brain function. Front Public Health. 4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Hasson U, Jovicich J, Robinson S. 2011. A neuronal basis for task-negative responses in the human brain. Cereb Cortex. 21:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M, Macdonald M, Lord C. 2013. Motor skills of toddlers with autism spectrum disorders. Autism. 17:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Monchi O, Albouy G, Jubault T, Ballarin E, Burnod Y, Doyon J. 2014. Striatal and hippocampal involvement in motor sequence chunking depends on the learning strategy. PLoS One. 9:e103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuzzi R, Murray MM, Maeder PP, Fornari E, Thiran J, Clarke S, Michel CM, Meuli RA. 2006. Visuo-motor pathways in humans revealed by event-related fMRI. Exp Brain Res. 170:472–487. [DOI] [PubMed] [Google Scholar]
- Mathias A, Grond F, Guardans R, Seese D, Canela M, Diebner HH. 2004. Algorithms for spectral analysis of irregularly sampled time series. J Stat Softw. 11:1–27. [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Landa RJ, Charman T, Stone WL, Constantino JN, et al. . 2013. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J Am Acad Child Adolesc Psychiatry. 52:300–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. 2009. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 132:2413–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. 1995. Mullen scales of early learning. Circle Pines (MN): Guidance Service Publishing. [Google Scholar]
- Murray GK, Veijola J, Moilanen K, Miettunen J, Glahn DC, Cannon TD, Jones PB, Isohanni M. 2006. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J Child Psychol Psychiatry. 47:25–29. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, Ferretti A, Gianni Perrucci M, Del Gratta C, Kakigi R, Luca Romani G. 2008. Somato-motor inhibitory processing in humans: an event-related functional MRI study. Neuroimage. 39:1858–1866. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Barber AD, Mostofsky SH. 2014. a. Precentral gyrus functional connectivity signatures of autism. Front Syst Neurosci. 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Choe AS, Barber AD, Pekar JJ, Mostofsky SH. 2016. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry. 79:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. 2014. b. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 35:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, Mckinstry RC, Raichle ME, Conturo TE. 1997. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 6:156–167. [DOI] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. 2012. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 36:901–942. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. . 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett JR Jr, Kandala S, Hoertel S, Snyder AZ, Elison JT, Nishino T, Feczko E, Dosenbach NU, Nardos B, Power JD, et al. . 2015. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Dev Cogn Neurosci. 12:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2015. The brain’s default mode network. Annu Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci USA. 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivals I, Personnaz L, Taing L, Potier MC. 2007. Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics. 23:401–407. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. 2001. The cortical motor system. Neuron. 31:889–901. [DOI] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. 2008. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA. 105:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco K, Cauda F, D’Agata F, Mate D, Duca D, Geminiani G. 2009. Reorganization and enhanced functional connectivity of motor areas in repetitive ankle movements after training in locomotor attention. Brain Res. 1297:124–134. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. . 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. 2010. Reward processing in autism. Autism Res. 3:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Merillat S, Jancke L. 2015. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage. 108:47–59. [DOI] [PubMed] [Google Scholar]
- Shank L. 2011. Mullen scales of early learning In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York (NY): Springer New York; p. 310. [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. 2011. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 56:1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube W, Mouthon M, Leukel C, Hoogewoud HM, Annoni JM, Keller M. 2015. Brain activity during observation and motor imagery of different balance tasks: an fMRI study. Cortex. 64:102–114. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Paul LK, Adolphs R. 2014. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb Cortex. 24:1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaina LM, Solomon J, Chowdhury S, Sinha P, Belliveau JW. 2001. Functional neuroanatomy of biological motion perception in humans. Proc Natl Acad Sci USA. 98:11656–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen M, Allali G, Rieger SW, Assal F, Vuilleumier P. 2014. The influence of individual motor imagery ability on cerebral recruitment during gait imagery. Hum Brain Mapp. 35:455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger M, Estevez N, Hepp-Reymond MC, Kiper D, Kollias SS, Eng K, Hotz-Boendermaker S. 2013. Enhanced activation of motor execution networks using action observation combined with imagination of lower limb movements. PLoS One. 8:e72403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C. 2004. An action perspective on motor development. Trends Cogn Sci. 8:266–272. [DOI] [PubMed] [Google Scholar]
- Wagner J, Stephan T, Kalla R, Bruckmann H, Strupp M, Brandt T, Jahn K. 2008. Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Exp Brain Res. 191:247–255. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, et al. , the IBIS network . 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, Strang J, Kenworthy L, Gaillard WD, Vaidya CJ. 2015. Default mode network segregation and social deficits in autism spectrum disorder: evidence from non-medicated children. Neuroimage Clin. 9:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapparoli L, Invernizzi P, Gandola M, Verardi M, Berlingeri M, Sberna M, De Santis A, Zerbi A, Banfi G, Bottini G, et al. . 2013. Mental images across the adult lifespan: a behavioural and fMRI investigation of motor execution and motor imagery. Exp Brain Res. 224:519–540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.