Abstract
Whereas the HIV prevalence in Guinea is among the lowest in Africa, many PLHIV in Guinea are malnourished. This study assessed the effect of a nutritional supplementation program on body mass index and CD4 count among adult PLHIV on ART. Study participants were PLHIV who came for consultation in the study sites between May and July 2016. The data came from two sources: retrospectively from participants’ medical records and interviews at the time of recruitment into the study. About six months before they were recruited into the study, some of the PLHIV started to receive a monthly nutritional supplementation consisting of Corn-Soy Blend and oil. Analytic methods included bivariate and multivariable methods. The intervention increased the mean BMI by 7.4% and the average current CD4 count by 4.7% compared to nonintervention (P<0.001). Programs in low resource settings should consider nutrition assistance as part of a comprehensive strategy to ensure optimal metabolic and immunological functions among PLHIV.
Key words: Nutritional supplementation, body mass index, CD4 count, HIV, Guinea
Introduction
The acquired immune deficiency syndrome (AIDS) is a public health problem in many parts of Africa. The disease, caused by the human immunodeficiency virus (HIV), weakens the body’s immune system and exposes the infected persons to opportunistic diseases. According to the 2012 Demographic and Health Survey, HIV prevalence rate was 1.7% in Guinea. As in most other African countries, women (2.1%) were almost twice as likely as men (1.2%) to be infected.1
As HIV progresses to AIDS, nutritional requirements in terms of proteins, micronutrients, and energy increase.2,3 Unfortunately, malnutrition is a common problem among people living with HIV (PLHIV).3-6 The reasons for this are multiple including the fact that AIDS is often associated with loss of appetite, nausea and vomiting (often due to malabsorption of nutrients) and limited financial resources due to job loss and inability to HIV unable to procure adequate nutrition.3,7,8 Among PLHIV, not only do malnutrition and HIV tend to co-occur, the two problems tend to have synergistic effects in such a way as to aggravate outcomes for PLHIV.9,10 A healthy and balanced diet helps to compensate for energy deficits due to AIDS, strengthen the immune system, and improve metabolic processes and treatment outcomes. 2,11 Furthermore, adequate nutrition helps to mitigate the pace of immune suppression in individuals infected with HIV and foster adherence to treatment.3,4 In contrast, inadequate nutrition and food insecurity tend to hasten disease progression and is associated with poorer treatment outcomes for PLHIV.10 For example, these conditions have been associated with lower CD4 counts and immune degradation,12,13 increased risk of transmission and metabolic syndrome,2 increased risk of opportunity infections,5 increased risk of mortality,5,14 poor adherence to ART15,16 and reduced quality of life.6
Whereas the problem of malnutrition in HIV infected people is more common and severe in Africa, especially in countries without universal access to antiretroviral treatment, malnutrition continues to be a common problem in developed countries and in developing countries with widespread access to ART.12,14,17,18 In recent years, considerable improvements have been accomplished both in our understanding of the link between HIV and malnutrition and in the identification of effective strategies to improve the situation. Many of the studies that have contributed to this improved understanding come from African countries.5,14,19 The World Health Organization has long acknowledged the importance of adequate nutrition for PLHIV and made specific recommendations about increased calorie intake as the disease progresses. 20 Nutrition-based interventions are becoming increasingly common in the care and support of PLHIV and have been found to be effective in Africa and other settings. 3,8,19,21,22 For example, in their study in Abuja, Amlogu, Tewfik found that provision of a nutritional functional meal made from locally available ingredients increase CD4 count by 40.8 cells/mm3 compared to a decrease of 18.12 cells/mm3 in the control group after a period of six months. Similarly in a double-blinded randomized control trial found an increase of 65 cells in the mean absolute CD4 count in the group that received micronutrient supplement compared to a decrease of 6 cells in the control group after 12 weeks of intervention.23-26
In Guinea, the number of PLHIV receiving ART have increased steadily over the years from 14,999 in 2009 to 27,792 in 2013.24 This increase notwithstanding, malnutrition and weight loss continue to be a major concern among PLHIV in Guinea. According to the 2014 survey of nutritional status and vulnerability of PLHIV on ART in Conakry and six other cities revealed a prevalence of malnutrition of 24.3% at the onset of treatment.25
The aim of this study was to assess the effect of nutritional supplementation on body mass index and CD4 count among adult PLHIV on antiretroviral therapy (ART) in Conakry Guinea.
Materials and Methods
Study population
Conakry, the capital of Guinea has the highest HIV prevalence rate in the country: 2.7%. With a population of 1.77 million, the city is by far the most populous and urbanized in the country. Conakry comprises five communes: Kaloum, Dixinn, Matam, Matoto and Ratoma. The study took place in the following health facilities: Centre Hospitalier Universitaire (CHU) Ignace Deen, CHU Donka, Centre Médical Communal (CMC) Matam, CMC les Flamboyants, Centre de Santé (CS) Gbessia Port 1, and CS Wanindara. These facilities are reference sites for HIV care and treatment in Conakry. The study targeted male and female PLHIV who obtained care from the study sites between April 2008 to January 2016. In parallel to the antiretroviral treatment, some of the participants were selected based on their BMI to receive a nutrition-based intervention. The intervention consisted of nutritional support. Specifically, underweight PLHIV (with BMI lower than 18.5) were provided with a monthly supply of a bag of fortified flour (Corn-Soya Blend) and five liters of vegetable oil for a period of six months. The duration of supplementation was six months for all patients who benefited the intervention. The duration of HAART depended on the time of diagnosis and HAART initiation.
Data collection
The data that we analyzed in this manuscript came from interviews with PLHIV who received HIV care in the study sites between May and July 2016. PLHIV aged at least 15 years and having been on treatment for at least three months were eligible for participation in a cross-sectional survey. The data was collected during face to face interview with PLHIV and was completed by baseline demographic and clinical data collected directly from patients’ medical records and covered the period from April 2008 to January 2016. The information extracted from the medical records included date diagnosed with HIV, date ART started, weight at the initiation of ART and CD4 count at the initiation of ART. Data were collected with a pretested survey tool that included questions on socio-demographic characteristics (sex, age, education level, marital status, and occupation); co-residence with another PLHIV, household size, prior and current experience with opportunistic infection, appetite-related problems, frequency of receiving nutritional supplementation, eating habits, and exposure to nutritional counseling at the health facility. Data collection also included obtaining the weight and height study participants and assessing their CD4 status. Participants were weighed with light clothing on using a high precision digital scale. Their height was measured using a measuring tape. The CD4 T cell count was based on an automated method (BD Bioscience, BD FACSCount™) using test strips.
Ethical and regulatory aspects
Ethical clearance for this study was obtained from the Scientific Committee of the Department of Public Health University of Conakry College of Medicine. Prior to conducting the interviews, field workers explained the objectives, procedures and associated risk and benefits of the study, and obtained informed consent from each respondent. Furthermore, the respondents were protected through individual face-toface interviews without a third-party, assurance of confidentiality and voluntary participation.
Analysis
We started by examining the differences in the socio-demographic characteristics of the study participants that received nutritional supplementation and those that did not. We then performed both unadjusted and multivariable regression analysis. Prior to conducting the analysis, we checked for normality of the dependent variables by examining the Kurtosis and skewness. The results suggested that the variables were not normally distributed. We therefore performed log-transformation of the variables before using them in the regression models. For the bivariate analysis, we examined the unadjusted relationship between the dependent variables and each of the independent variables using linear regression. The variables that were significant at 0.2 level were retained for inclusion in a multivariable linear regression. For the multivariable regression, we report both the regression coefficients and the beta weights. All analyses were performed in Statistical Package for Social Science (SPSS) version 24.26
Results
Socio-demographic characteristics of study participants
The sample included 450 study participants; 138 of these were in the intervention group (that is, they received nutritional supplementation) while 312 were in the nonintervention group. The differences in the socio-demographic and baseline nutritional characteristics between the two groups are presented on Table 1. Overall, the sample included 26.9% men and 73.1% women; there were no significant differences in sex distribution between the intervention and non-intervention groups. Similarly, the two groups did not differ by marital status, education level or household size. Furthermore, the two groups did not differ by age, treatment duration, CD4 at initiation of treatment. In contrast, there were significant differences between the two groups in terms of exposure to nutritional counseling during facility visit. Specifically, the participants in the intervention group (61.6%) were more likely to have received nutritional counseling than their peers in the non-intervention group (45.2%). Similarly, the participants in the intervention group (57.2%) were more likely than their peers in the non-intervention group (44.2%) to have recently experienced an opportunistic infection. Problems with appetite were more prevalent in the non-intervention (25.3%) than in the intervention (16.7%) groups. Furthermore, the participants in the intervention group (39.1%) were more likely than their peers in the non-intervention group (25.3%) to have an income lower than 8175 Guinean Francs. Finally, the two groups differed significantly in terms of BMI at initiation of treatment; the average BMI was 16.63 in the intervention group and 20.95 in the non-intervention group.
Table 1.
Baseline (at ART initiation) characteristics of intervention and non-intervention groups.
| Independent Variable/Category | Intervention (n=138) | Non-Intervention (n=312) | P | ||
|---|---|---|---|---|---|
| N. | % mean/median | N. | % mean/median | ||
| Median BMI at treatment initiation | 138 | 16.78 | 312 | 20.69 | <0.001 |
| Sex | |||||
| Male | 32 | 23.2 | 89 | 28.5 | 0.239 |
| Female | 106 | 76.8 | 223 | 71.5 | |
| Mean current age in years | 138 | 35.8 | 312 | 37.3 | 0.118 |
| Education level | |||||
| No education | 85 | 61.6 | 177 | 56.7 | 0.566 |
| Primary | 17 | 12.3 | 40 | 12.8 | |
| Middle School | 16 | 11.6 | 32 | 10.3 | |
| High School | 14 | 10.1 | 37 | 11.8 | |
| Tertiary | 6 | 4.3 | 26 | 8.3 | |
| Marital Status | |||||
| Polygynous marriage | 62 | 44.9 | 116 | 37.2 | 0.401 |
| Monogamous marriage | 24 | 17.4 | 70 | 22.4 | |
| Divorced/widowed | 26 | 18.8 | 67 | 21.5 | |
| Never Married | 26 | 18.8 | 59 | 18.9 | |
| Average treatment duration in months | 138 | 42.0 | 312 | 43.9 | 0.257 |
| Household size | |||||
| Five members or fewer | 85 | 61.6 | 180 | 57.7 | 0.438 |
| At least six members | 53 | 38.4 | 132 | 42.3 | |
| Nutrition counseling | |||||
| Did not receive | 53 | 38.4 | 171 | 54.8 | <0.001 |
| Received | 85 | 61.6 | 141 | 45.2 | |
| Recent or current opportunistic infection | |||||
| Did not have | 59 | 42.7 | 174 | 55.8 | 0.011 |
| Had | 79 | 57.3 | 138 | 44.2 | |
| Recent or current appetite-related problems | |||||
| Did not have | 115 | 83.3 | 233 | 74.7 | 0.043 |
| Had | 23 | 16.7 | 79 | 25.3 | |
| Poverty | |||||
| Non-poor (Income at least 8175 Guinean Francs per day) | 84 | 60.9 | 233 | 74.7 | 0.003 |
| Poor (Income less than 8175 Guinean Francs per day) | 54 | 39.1 | 79 | 25.3 | |
| Another household member living with HIV | |||||
| No | 95 | 68.8 | 214 | 68.6 | 0.958 |
| Yes | 43 | 31.2 | 98 | 31.4 | |
Effects of nutritional supplementation on BMI
The median BMI increased by 4.7 between treatment initiation and end of the study among the intervention group compared to 1.9 among the non-intervention (P<0.001). Results of the bivariate and multivariable linear regression models are presented on Table 2. The bivariate results show a negative association between exposure to the intervention and BMI at the end of the study. Specifically, the BMI was about 6% [exp(-0.06)-1] smaller among intervention participants compared to the control. The relation of BMI at start of treatment and current BMI was strong and positive. Other variables positively associated with current BMI include being currently or previously married compared to never married, treatment duration in months and coresidence with another HIV-positive person. In contrast, being male, education level, recent episode of opportunistic infections, problems with appetite and poverty were negatively associated with current BMI. Household size and receiving nutritional counseling during facility visits were not significantly associated with current BMI.
Table 2.
Results of the regression of log-transformed BMI on lagged value of BMI, intervention participation status and other variables Conakry 2016.
| Independent Variable/Category | Unadjusted Coefficient/SE1,2 | Adjusted Coefficient/SE2,3 | Beta |
|---|---|---|---|
| Received nutritional supplementation (RC = Did not receive) | -0.060** (.020) | 0.071*** (0.018) | 0.166 |
| BMI at treatment initiation | 0.031*** (0.002) | 0.031*** (0.002) | 0.594 |
| Male sex (RC = female sex) | -0.071*** (0.021) | -0.061*** (0.018) | -0.137 |
| Current age | 0.0024** (0.0009) | 0.002** (0.0008) | 0.105 |
| Education level (RC = No education) | |||
| Primary | 0.001 (0.029) | 0.009 (0.021) | 0.015 |
| Middle School | -0.080** (0.031) | -0.044§ (0.023) | -0.069 |
| High School | -0.048 (0.030) | 0.0009 (0.023) | 0.001 |
| Tertiary | -0.048 (0.037) | -0.003 (0.029) | -0.005 |
| Marital Status (RC = Polygynous marriage) | |||
| Monogamous marriage | -0.027 (0.024) | -0.024 (0.019) | -0.049 |
| Divorced/widowed | -0.061* (0.025) | -0.026 (0.021) | -0.055 |
| Never Married | -0.088*** (0.025) | -0.015 (0.021) | -0.029 |
| Treatment duration in months | 0.0008** (0.0003) | 0.0004§ (0.0002) | 0.064 |
| Household size at least six members (RC = five members or fewer) | 0.010 (0.019) | - | - |
| Received nutrition counselling (RC = Did not receive) | 0.019 (0.019) | - | - |
| Recently or currently had an opportunistic infection (RC = Did not have) | -0.092*** (0.018) | -0.043** (0.014) | -0.109 |
| Recently or currently had appetite-related problem (RC = Did not have) | -0.135*** (0.021) | -0.118*** (0.016) | -0.252 |
| Poor (RC = non-poor) | -0.044* (0.020) | -0.021 (0.015 | -0.049 |
| Another household member living with HIV | 0.054** (0.020) | 0.010 (0.016) | 0.024 |
| (RC = No Other household member living with HIV) | |||
| Explained variance (R2) | 47.2% | - |
1Effects not adjusted for other covariates; 2the dependent variable is log-transformed; therefore the coefficients should be converted to percent change for better understanding and correct interpretation: Percent Change = (exp(Coefficient) – 1)*100. 3Effects adjusted for other covariates.
§P<0.1
*P<0.05
**P<0.01
***P<0.001
The multivariable logistic results show that the independent variables included in the model collectively predict 47.2% of the variance in current BMI. After controlling for BMI at treatment initiation and other variables that are likely to affect current BMI, the intervention has significant effects on current BMI. Specifically, compared to non-intervention, the intervention was associated with a 7.4% [exp(.0712328)-1] increase in current BMI. A unit increase in baseline BMI increases the average current BMI by 3.1% while being male decreases the measure by 5.9% compared to being female. A one-year increase in age increases current BMI by 0.2%. In contrast, current recent episode of an opportunistic infection decreases the current BMI at end of study by 4.2% while problem with appetite decreases it by 11.2%. The effects of treatment duration and education were only marginally significant whereas, marital status, poverty and co-residence with another HIVpositive person were not at all significant predictors. A look at the beta weights indicate that the strongest predictors of BMI at end of study are BMI at treatment initiation (a reflection of stability effects), problems with appetite, and the intervention.
Effects of nutritional supplementation on CD4 count
Between treatment initiation and end of the study, the median CD4 count increased by 266.5 cells/mm3 in the intervention group while the increase was 163 cells/mm3 in the non-intervention group (P<0.001) (Figure 1). Results of the bivariate analysis (unadjusted regression) indicate that the intervention has no significant effects on current CD4 count (Table 3). Similarly, current CD4 count was not significantly related to current age, education, marital status, household size, problems with appetite, and poverty. In contrast, CD4 count at treatment initiation, sex, treatment duration, recent or current experience with opportunistic infections, and exposure to nutrition counseling during facility visits were significantly associated with current CD4 count. The association of current CD4 count with coresidence with another HIV-positive person was only marginally significant.
Figure 1.
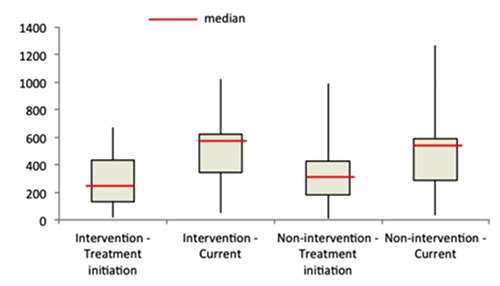
Change in CD4 count by intervention status, treatment initiation and end of study.
Table 3.
Results of the regression of log-transformed CD4 count on lagged value of CD4 count, intervention participation status and other variables, Conakry 2016.
| Independent Variable/Category | Unadjusted Coefficient/SE1,2 | Adjusted Coefficient/SE2,3 | Beta |
|---|---|---|---|
| Received nutritional supplementation (RC = Did not receive) | 0.031 (0.022) | 0.046** (0.017) | 0.097 |
| CD4 count at treatment initiation | 0.001***(0.00004) | 0.0007*** (0.00004) | 0.614 |
| Male sex (RC = female sex) | -0.049* (0.023) | -0.003 (0.018) | -0.005 |
| Current age | 0.0007 (0.001) | - | - |
| Education level (RC = No education) | |||
| Primary | -0.042 (0.032) | - | |
| Middle School | -0.027 (0.034) | - | |
| High School | -0.011 (0.034) | ||
| Tertiary | 0.041 (0.041) | ||
| Marital Status (RC = Polygynous marriage) | |||
| Monogamous marriage | -0.020 (0.028) | - | - |
| Divorced/widowed | -0.006 (0.028) | ||
| Never Married | -0.001 (0.029) | ||
| Treatment duration in months | 0.001*** (0.0003) | 0.0007** (0.0003) | 0.094 |
| Household size at least six members (RC = five members or fewer) | 0.005 (0.021) | - | - |
| Received nutrition counseling (RC = Did not receive) | 0.080***(0.020) | 0.048** (0.16) | 0.111 |
| Recently or currently had an opportunistic infection (RC = Did not have) | -0.063** (0.020) | -0.001 (0.016) | -0.002 |
| Recently or currently had appetite-related problem (RC = Did not have) | -0.021 (0.025) | - | - |
| Poor (RC = non-poor) | -0.001 (0.023) | - | - |
| Another household member living with HIV | 0.039§ (0.022) | 0.014 (0.017) | 0.031 |
| (RC = No Other household member living with HIV) | |||
| Explained variance (R2) | - | 42.2% | - |
1Effects not adjusted for other covariates; 2the dependent variable is log-transformed; therefore the coefficients should be converted to percent change for better understanding and correct interpretation: Percent Change = (exp(Coefficient) –1)*100. 3Effects adjusted for other covariates.
§P<0.1
*P<0.05
**P<0.01
***P<0.001
The multivariate results show that the seven explanatory variables jointly explain 42.2% of the variance in current CD4 count. The results further show that the significant predictors of current CD4 count are CD4 count at treatment initiation, intervention participation, treatment duration, and exposure to nutrition counseling during facility visits. Specifically, the higher the CD4 at treatment initiation, the higher the current CD4 cunt is: a one-point increase in CD4 at treatment initiation increases current CD4 by 0.07%. The effect of the intervention is strong and positive such that being in the intervention group increases the average current CD4 count by 4.7% compared to the non-intervention group. The effects of nutrition counseling and length of time in treatment were positive. The beta coefficients reveal that the most important predictors of current CD4 count were CD4 count at treatment initiation, nutrition counseling and the intervention.
Discussion
This manuscript examined the role of a nutrition-based intervention on current BMI and CD4 count among PLHIV on ART in Conakry, Guinea. The results indicate that the intervention has net positive effects on both outcomes. This finding echoes what multiple other studies in Africa and elsewhere have found.3,8,19,21,23 This finding underscores the need for including nutritional assistance as part of a comprehensive strategy for promoting healthy outcomes among PLHIV on treatment. Another important finding from this study is that after controlling for intervention status and other variables associated with metabolic and immunological functions the lagged values of BMI and CD4 (at time of treatment initiation) are the strongest predictors of the current values. Substantively, this finding indicates the need to start treatment early before metabolic and immunologic functions are compromised, echoing suggestions from prior studies.27,28
This study further found differences and communalities among the predictors of current BMI and CD4 count. Both outcomes were sensitive to the intervention and lagged value of the outcome. Neither marital status nor education predicted current BMI or CD4. Current age, sex, opportunistic infections and appetite-related problems strongly predicted current BMI but not current CD4 count. In contrast, treatment duration and nutritional counseling were significantly associated with CD4 count but not with BMI. The reasons for these differences in predictors are not entirely clear. Nonetheless, it probably makes sense that opportunistic infections and appetite-related problems strongly predict current BMI, an outcome sensitive to short-term changes in health conditions. The positive relationship of CD4 count with treatment duration makes intuitive sense since engagement in treatment is expected to lead to viral suppression and increased CD4 count, eventually.
Our study has several limitations including the fact that participants were not randomly allocated to intervention and nonintervention groups. Rather, the selection privilege those with lower income and lower BMI at treatment initiation. It is therefore prone to selection bias. Nonetheless, our results suggest that both income and BMI are good criteria for targeted nutritional supplementation programs in resource-constrained settings. Furthermore, data on treatment initiation and other baseline data were collected retrospectively, which might have led to missing values and limited the range of predictors that we could have included in the estimated models. For example, time to nutritional supplementation after treatment initiation and compliance with nutritional supplementation were not accounted for in the analyses. Finally, because the sampling was not stratified by municipality, the study does not allow comparison of the outcomes across municipalities. All the same, this study, the first of its kind in Guinea, fills an existing gap and adds to the growing body of literature on the benefits of nutritional supplementation. The 2014/2015 Ebola outbreak has weakened the Guinean health system, decreased the performance of programs and services, and compromised community confidence in the health system.29,30 The findings can be used to support efforts designed to strengthen the health system and boost public confidence in the system.
Conclusions
This study demonstrated that nutritional supplementation has a beneficial effect on BMI and CD4 count among adults on antiretroviral therapy (ART) in Conakry, Guinea. The positive effects of the nutritional supplement, comprising enriched flour (Corn-Soya Blend) and vegetable oil Programs, remained strong after controlling for other variables that are known to influence BMI and CD4 count. Programs in low resource settings should consider nutrition assistance as part of a comprehensive strategy to ensure optimal metabolic and immunological functions among PLHIV.
Acknowledgements
The authors acknowledge the collaboration of the personal of the PLHIV care services of the Centre Hospitalo- Universitaire (CHU) d’Ignace Deen, CHU de Donka, Centre Médical Communal (CMC) de Matam, CMC les Flamboyants, Centre de Santé (CS) de Wanindara and CS de Gbessia Port 1 that made the implementation of this study possible. We are also grateful to the Programme National de Prise en Charge Sanitaire et de Prévention des IST/VIH/SIDA (PNPCSP), Comité National de Lutte contre le SIDA (CNLS) and Gamal University, Conakry for their support in the implementation of this study.
Funding Statement
Funding: none.
References
- 1.Institut National de la Statistique (INS) et ICF International. Enqunque known to influence BMI and CD4 count. Programs in low resource sCalverton, Maryland, USA; 2012. [Google Scholar]
- 2.Friis H, Olsen MF, Filteau S. Nutrition and HIV. International encyclopedia of public health. Academic Press, Incorporated; 2017. [Google Scholar]
- 3.Byron E, Gillespie S, Nangami M. Integrating nutrition security with treatment of people living with HIV: lessons from Kenya. Food Nutr Bull 2008;29:87:87. [DOI] [PubMed] [Google Scholar]
- 4.Duggal S, Chugh TD, Duggal AK. HIV and Malnutrition: Effects on Immune System. Clin Dev Immunol 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull 2010;31:S313-44. [PubMed] [Google Scholar]
- 6.Tesfaye M, Kaestel P, Olsen MF, et al. Food insecurity, mental health and quality of life among people living with HIV commencing antiretroviral treatment in Ethiopia: a cross-sectional study. Health Qual Life Outcomes 2016;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe P, Juste MAJ, Ambroise A, et al. Early Versus Standard Antiretroviral Therapy for HIV Infected Adults in Haiti. N Engl J Med 2010;363:257-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alo C, Ogbonnaya LU, Azuogu BN. Effects of nutrition counseling and monitoring on the weight and hemoglobin of patients receiving antiretroviral therapy in Ebonyi State, Southeast Nigeria. HIVAIDS Auckl NZ 2014;6:91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedle D, Gelaw B, Muluye D, Mesele M. Prevalence of malnutrition and its associated factors among adult people living with HIV/AIDS receiving antiretroviral therapy at Butajira Hospital, southern Ethiopia. BMC Nutr 2015;1:5. [Google Scholar]
- 10.PhD MN. The risk of developing malnutrition in people living with HIV/AIDS: Observations from six support groups in Botswana. South Afr J Clin Nutr 2009;22:89-93. [Google Scholar]
- 11.Ivers LC, Cullen KA, Freedberg KA, et al. HIV/AIDS, Undernutrition, and Food Insecurity. Clin Infect Dis 2009;49:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis Off Publ Infect Dis Soc Am 2006;42:836-42. [DOI] [PubMed] [Google Scholar]
- 13.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutr Burbank Los Angel Cty Calif 2005;21:96-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu E, Spiegelman D, Semu H, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis 2011;204:282-90. [DOI] [PubMed] [Google Scholar]
- 15.Young S, Wheeler AC, McCoy SI, Weiser SD. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav 2014;18:S505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiser SD, Tuller DM, Frongillo EA, et al. Food Insecurity as a Barrier to Sustained Antiretroviral Therapy Adherence in Uganda. PLoS One 2010;5:e10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Highleyman L. Nutrition and HIV. BETA 2006;18;18-32. [PubMed] [Google Scholar]
- 18.Anema A, Weiser SD, Fernandes KA, et al. High prevalence of food insecurity among HIV-infected individuals receiving HAART in a resource-rich setting. AIDS Care 2011;23:221-30. [DOI] [PubMed] [Google Scholar]
- 19.Amlogu AM, Tewfik S, Wambebe C, Tewfik I. A comparative study: long and short term effect of a nutrition sensitive approach to delay the progression of HIV to AIDS among people living with HIV (PLWH) in Nigeria. Funct Foods Health Dis 2016;6:79-90. [Google Scholar]
- 20.World Health Organization. Nutrient requirements for people living with HIV/AIDS. Geneva, Switzerland; 2004. Available from: http://www.who.int/iris/handle/10665/42853 [Google Scholar]
- 21.Amlogu AM, Tewfik S, Wambebe C, et al. Tailored Functional Recipe (TFR) Approach to Delay the Progression of HIV to AIDS among People Living with HIV (PLWH) in Abuja, Nigeria. Pharmacol Amp Pharm 2014;5:926. [Google Scholar]
- 22.Ivers LC, Chang Y, Gregory Jerome J, Freedberg KA. Food assistance is associated with improved body mass index, food security and attendance at clinic in an HIV program in central Haiti: a prospective observational cohort study. AIDS Res Ther 2010;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser JD, Campa AM, Ondercin JP, et al. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: a prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr 2006;15:523. [DOI] [PubMed] [Google Scholar]
- 24.Ministnistmune Defic Syndr 1999. 2006 Aug 15;42(5):523 Baum MK. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretrov [DOI] [PubMed] [Google Scholar]
- 25.Programme Alimentaire Mondial et Centre National de Lutte contre le Sida (PAM/CNLS). Evaluation du statut nutritionnel et de la vulnérabilité des PVVIH sous traitement ARV et des femmes suivies en Guinée. 2014. [Google Scholar]
- 26.IBM 32. IBM Corporation. SPSS Statistics 24 Brief Guide. Armonk, NY: IBM Corporation; 2016. [Google Scholar]
- 27.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Group TTA 12136 S. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015;373:808. [DOI] [PubMed] [Google Scholar]
- 29.Delamou A, Ayadi AME, Sidibe S, et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health 2017;5:e448-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brolin Ribacke KJ, Saulnier DD, Eriksson A, von Schreeb J. Effects of the West Africa Ebola Virus Disease on Health-Care Utilization – A Systematic Review. Front Public Health 2016;4: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]


