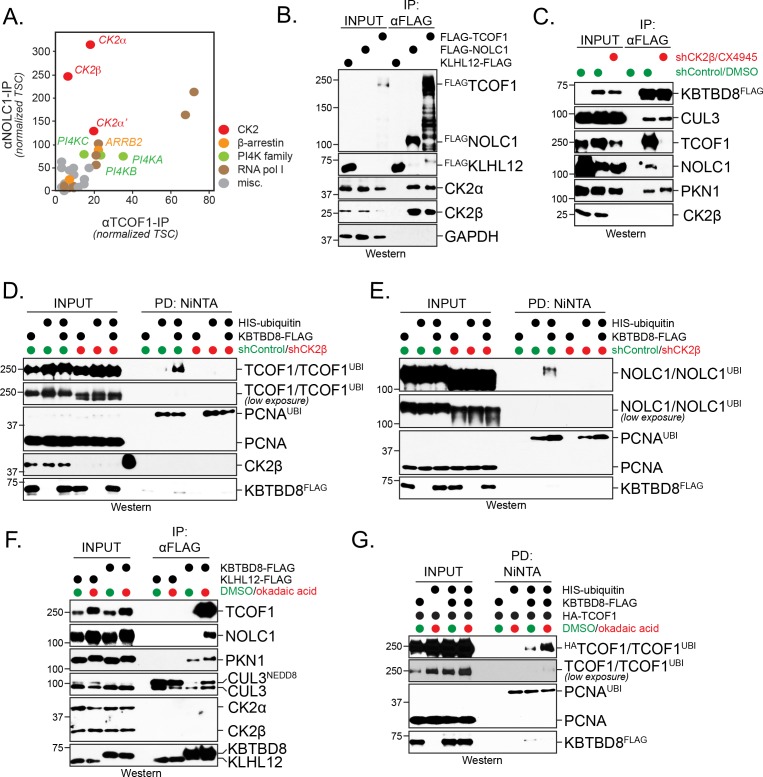
Figure 1. CK2 kinase is required for CUL3KBTBD8-substrate binding and ubiquitylation in cells.
(A) Both NOLC1 and TCOF1 associate with the CK2 kinase. FLAGNOLC1 and FLAGTCOF1 were affinity-purified from 293 T cells and specific binding partners were determined by CompPASS mass spectrometry. Total spectral counts (TSCs) of specific interactors were normalized to 1000 TSCs for each bait protein and plotted against each other. For each CUL3KBTBD8-substrate, three independent affinity-purification and mass spectrometry experiments were performed. The results of each affinity experiments were compared to a database, which included ~100 unrelated affinity-purifications performed with the same antibody and in the same cell line. (B) TCOF1 and NOLC1 associate with endogenous CK2 in cells. FLAGNOLC1 and FLAGTCOF1 were affinity-purified from 293 T cells, and co-purifying proteins were identified by western blotting using the indicated antibodies. KLHL12FLAG was used as a negative control. The low molecular weight species seen in TCOF1 samples are proteolytic degradation products caused by partial cleavage of the large and intrinsically disordered TCOF1 during the affinity-purification. (C) CK2 is required for substrate recognition by CUL3KBTBD8 in cells. 293 T cells were depleted of CK2β using shRNAs and the activity of the remaining CK2 kinase was simultaneously inhibited using the small molecule CX4945. KBTBD8FLAG, the substrate adaptor of CUL3, was affinity-purified and bound endogenous proteins were detected by western blotting using the indicated antibodies. PKN1 is a specific binding partner of KBTBD8 that is not known to be ubiquitylated by CUL3KBTBD8 and that is not important for neural crest specification. (D) CK2 is required for monoubiquitylation of TCOF1 in cells. Ubiquitylated proteins were purified under denaturing conditions from 293 T cells expressing HISubiquitin. Ubiquitylated proteins were detected by western blotting using the indicated antibodies. PCNA is ubiquitylated by a distinct E3 ligase and hence is used as a control for general ubiquitylation efficiency; unmodified PCNA is not detected in NiNTA pulldowns, demonstrating the specificity of this assay. Input: 3% of lysate used for denaturing purification. (E) CK2 is required for monoubiquitylation of NOLC1 in cells. Ubiquitylated NOLC1 was purified both in the absence or presence of CK2 inhibitors, as described for TCOF1 above. Input: 3% of lysate used for denaturing purification. (F) Inhibition of PP1 and PP2A phosphatases, which counteract CK2 in cells, promotes substrate binding by CUL3KBTBD8. KBTBD8FLAG or the negative control KLHL12FLAG were affinity-purified from cells treated with the PP1/PP2A-inhibitor okadaic acid. Co-purifying proteins, including endogenous TCOF1, NOLC1, and the control KBTBD8-interactor PKN1, were detected by western blotting. This experiment was performed at smaller scale than Figure 1C, explaining the lack of TCOF1 binding to KBTBD8 under conditions without phosphatase inhibitors. (G) Inhibition of PP1/PP2A phosphatases increases TCOF1 ubiquitylation in cells. Ubiquitylated HATCOF1 was purified under denaturing conditions from 293 T cells expressing HISubiquitin and treated with or without okadaic acid. Ubiquitylated proteins were detected by Western blotting. Input: 3% of lysate used for denaturing purification.