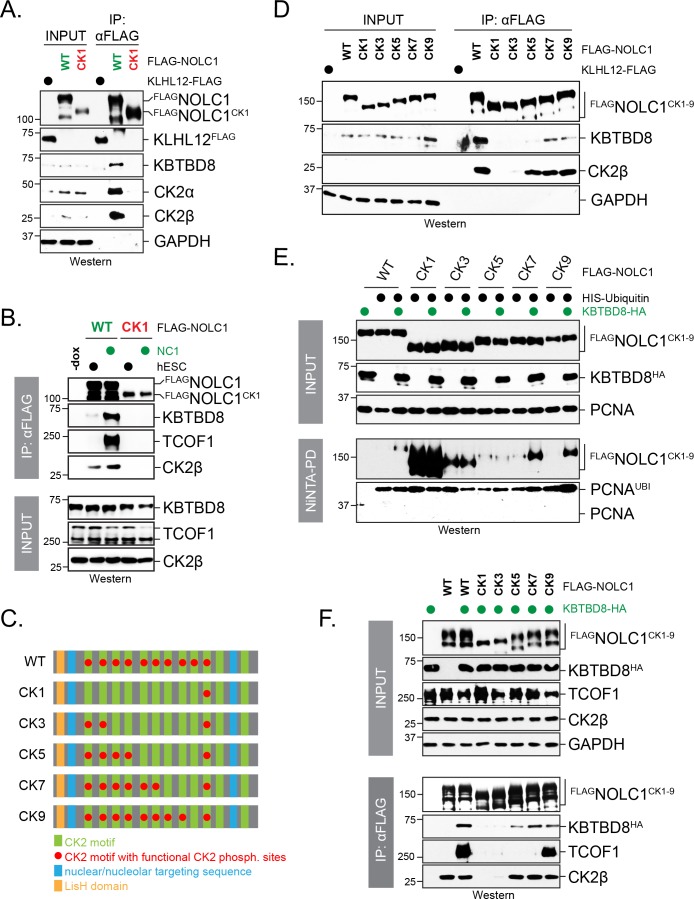
Figure 6. Multisite dependency of CUL3KBTBD8 substrate recognition in cells.
(A) A single CK2 motif is not sufficient for substrate recognition by KBTBD8 in cells. Wild-type FLAGNOLC1 or a mutant containing only a single CK2 motif (FLAGNOLC1CK1) were affinity-purified from 293 T cells and analyzed for binding to endogenous KBTBD8 and CK2 by western blotting using specific antibodies. (B) A single CK2 motif is not sufficient for KBTBD8 and TCOF1 recognition by NOLC1 in differentiating hESCs. FLAGNOLC1 was affinity-purified from hESCs or hESCs subjected to 1 day of neural conversion (NC1), and bound endogenous KBTBD8, TCOF1, or CK2 were detected by western blotting using specific antibodies. (C) Scheme of CK2-mutants of NOLC1. CK2 motifs that remained functional are labeled with a red dot; the last two CK2 motifs of NOLC1 do not mediate KBTBD8-binding in vitro, as shown above. (D) KBTBD8-recognition of NOLC1 depends on multiple CK2 motifs. The indicated NOLC1 variants were affinity-purified from 293 T cells and analyzed for binding to endogenous KBTBD8 or CK2β by western blotting using specific antibodies. Note that wild-type NOLC1, which contains all 10 functional CK2 motifs is the most efficient KBTBD8-recruiter. (E) Multisite dependency of NOLC1 ubiquitylation in cells. Ubiquitylated NOLC1 mutants were purified under denaturing conditions from 293 T cells that expressed HISubiquitin and, where indicated, KBTBD8HA. Ubiquitylation of FLAGNOLC1 was analyzed by αFLAG-Western; note that NOLC1 mutants containing up to five CK2 motifs are ubiquitylated by an E3 ligase distinct from KBTBD8 (i.e. ubiquitylation is observed in the absence of KBTBD8 expression in these cells). Ubiquitylation of endogenous PCNA was monitored by western blotting using specific antibodies. (F) Multisite dependency of TCOF1-NOLC1 complex formation. FLAGNOLC1 variants with increasing numbers of CK2 motifs were affinity-purified from 293 T cells that expressed KBTBD8 as indicated. Complex formation with endogenous TCOF1 was monitored by western blotting using specific antibodies. In addition, overexpression of KBTBD8 allowed for KBTBD8 recognition already in the presence of five CK2 motifs, consistent with multisite dependency providing an increase in affinity (i.e. that can in part be overcome by overexpression), rather than creating a specific complex E3 recognition element.