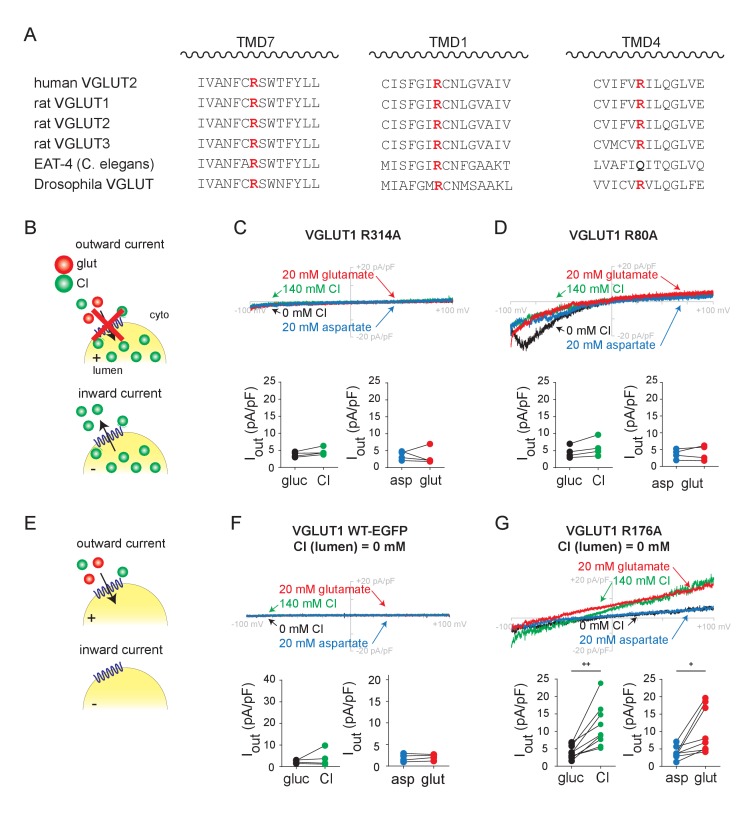
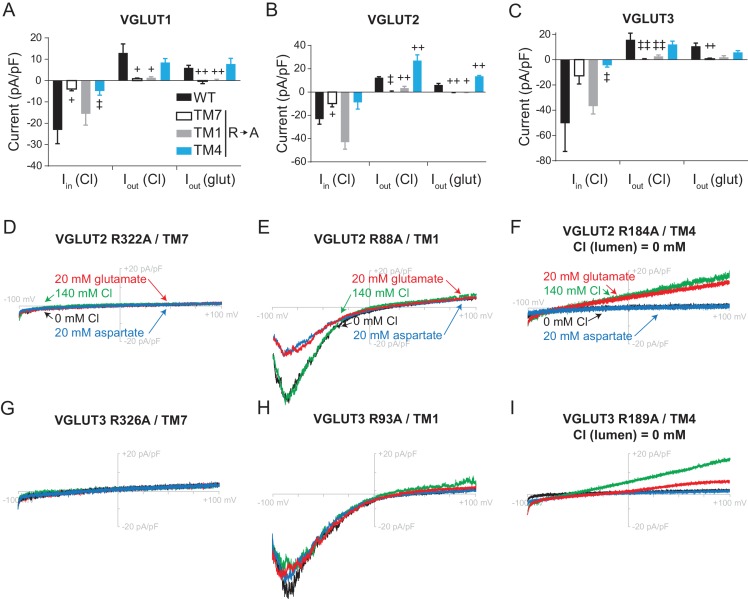
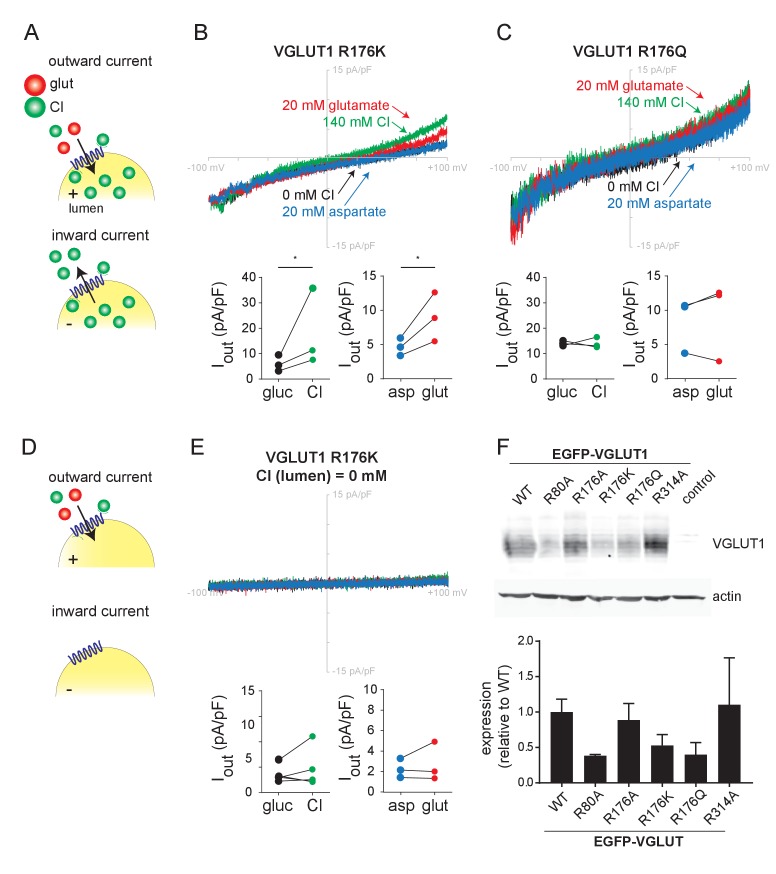
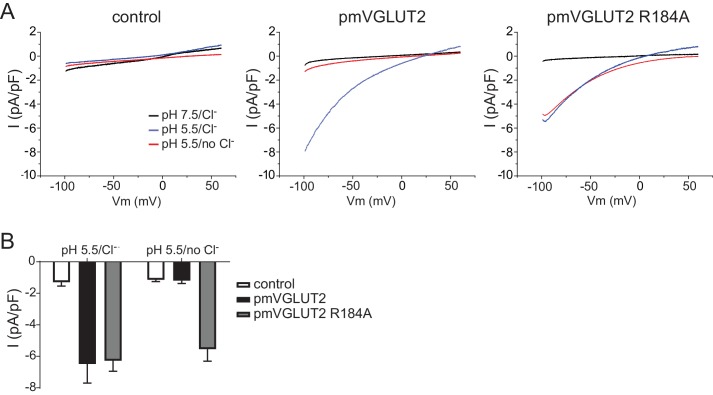
Figure 3. Transmembrane arginine residues control allosteric activation and permeation by Cl-.
(A) Sequence alignment of transmembrane domains TM 7, 1 and 4 from the related VGLUT isoforms and species with highly conserved arginine highlighted (red). (B) Representative whole endosome recordings (with 140 mM NMDG Cl at pH 5.0 in the pipette) of VGLUT1 R314A (C) and R80A (D) (n = 4 each). (E) Representative recordings with 0 mM Cl- in the pipette of endosomes expressing VGLUT1 WT (F) and R176A (G) (n = 5–9). Insets show maximum outward currents in the different external solutions (C, ICl, p=0.547, Iglut, p=0.080 both by paired t-test, D, ICl, p=0.062, Iglut, p=0.782 both by paired t-test, F, ICl, p=0.625 by Wilcoxon, Iglut, p=0.397 by paired t-test, G, ICl, p=0.002, Iglut, p=0.017 both by paired t-test). +p<0.05 and ++p<0.01 by paired t-test. Insets (C,D,F,G) indicate maximal outward currents for each endosome.