Key Points
BATs differ significantly in sensitivity for detecting menorrhagia in women with low VWF.
Despite pregnancy-related increases in plasma VWF levels, significant PPH may occur in women with low VWF.
Abstract
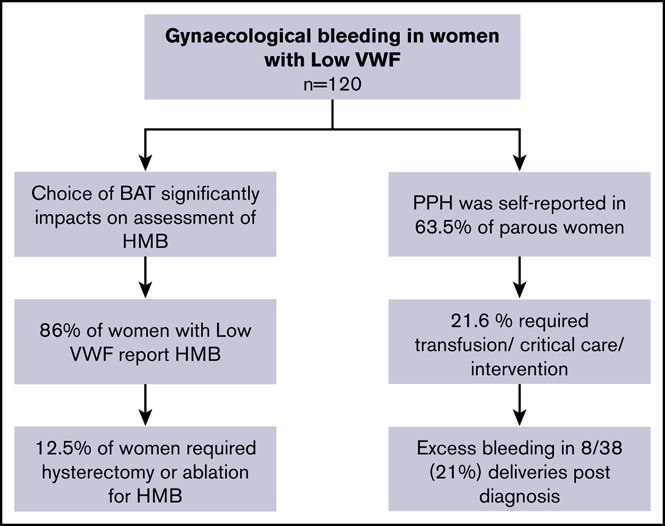
Gynecological bleeding is frequently reported in women with von Willebrand disease (VWD). Low von Willebrand factor (VWF) may be associated with significant bleeding phenotype despite only mild plasma VWF reductions. The contribution of gynecological bleeding to this phenotype has yet to be described. The optimal clinical bleeding assessment tool (BAT) to evaluate bleeding remains unclear. Using a standardized approach to phenotypic assessment, we evaluated gynecological bleeding and directly compared the Condensed Molecular and Clinical Markers for the Diagnosis and Management of type 1 VWD (Condensed MCMDM-1 VWD) and International Society on Thrombosis and Haemostasis (ISTH) BAT scores in 120 women enrolled in the Low von Willebrand in Ireland Cohort study. Heavy menstrual bleeding (HMB) was reported in 89% of female participants; 45.8% developed iron deficiency. Using identical data, Condensed MCMDM-1 VWD menorrhagia domain scores were significantly lower than ISTH BAT scores (2 vs 3; P < .0001), the discrepant results related to 40% of women not seeking medical consultation for HMB, reducing the sensitivity of the Condensed score. For those who reported HMB to physicians, the low VWF diagnosis was not expedited (age at diagnosis 34.2 vs 33.4 years in women failing to present; P = .7). Postpartum hemorrhage (PPH) was self-reported in 63.5% of parous women (n = 74); 21.6% required transfusion, critical care, radiological, or surgical intervention. Our data demonstrate that gynecological bleeding is frequently reported in women with low VWF; despite pregnancy-related increases in plasma VWF levels, these women may experience PPH. Defining the optimal management approach for these patients requires further research. This trial was registered at www.clinicaltrials.gov as #NCT03167320.
Visual Abstract
Introduction
An association between gynecological bleeding and von Willebrand disease (VWD) is well established. Indeed, the original index case described by Erik von Willebrand in 1926 was that of a young woman who died of fatal bleeding associated with her fourth menstrual period.1 Subsequent studies have confirmed that menorrhagia constitutes the most common reported bleeding symptom in women with type 1 VWD.2 Independent cohort studies have estimated that heavy menstrual bleeding (HMB) affects >75% of women with VWD3-6 resulting in iron-deficiency anemia; it is associated with a significant reduction in quality of life.7 For women presenting with HMB, previous studies have consistently demonstrated that reduced von Willebrand factor (VWF) levels is a common contributing factor in up to 20% of cases.8 Thus, not only is HMB common in women with VWD, but underlying VWD is also common in patients presenting with HMB.
Gynecological bleeding may also occur following delivery in the form of postpartum hemorrhage (PPH). This bleeding can be either in the form of a primary PPH (occurring in the initial 24 hours following delivery) or a secondary PPH (occurring during the subsequent 6-week postpartum period). As with HMB, PPH is also associated with significant increased maternal morbidity. Furthermore, several studies have reported significantly increased PPH rates (particular secondary PPH), in women with VWD compared with the normal population, with overall incidence rates ranging from 6% to 16%.9-13
Partial quantitative deficiencies of functionally normal VWF account for ∼75% of VWD cases.14 The US National Heart, Lung, and Blood Institute (NHLBI) and United Kingdom Hemophilia Centre Doctors’ Organization (UKHCDO) both recently proposed that patients with reduced plasma VWF levels and bleeding phenotypes should be considered in 2 distinct subsets.12,15 Patients with more marked reductions in plasma VWF levels (<30 IU/dL) are likely to have VWF gene mutations and should be labeled “type 1 VWD.” In contrast, patients with intermediate plasma VWF antigen (VWF:Ag) levels (30-50 IU/dL) are less likely to have VWF gene sequence variations and should be considered in a distinct category labeled “low VWF levels.” With respect to his latter cohort, the NHLBI guidelines further emphasized the need for more clinical information to better elucidate the relationship between low VWF levels and bleeding phenotype. Despite the evidence demonstrating significantly enhanced gynecological bleeding in women with significantly reduced VWF levels (<30 IU/dL), the relevance of more mild reductions in plasma VWF in this context has not previously been investigated. In this paper, we have used the Low von Willebrand in Ireland Cohort (LoVIC) study recently reported by Lavin et al16 to specifically investigate, for the first time, gynecological bleeding in female patients with low VWF levels.
Methods
Participants and clinical data
Participants were enrolled as part of the LoVIC study at a single center, the National Coagulation Centre, St. James’s Hospital, Dublin. Ethical approval was obtained from the local research ethics committee and written informed consent was obtained from participants prior to enrollment. Patients were eligible to enroll if they were ≥18 years of age with a confirmed diagnosis of low VWF, defined as a personal bleeding history and plasma VWF levels (VWF:Ag, VWF ristocetin cofactor [VWF:RCo], or VWF collagen-binding [VWF:CB] assay) in the 30 to 50 IU/dL range on 2 occasions, at least 3 months apart. All female enrollees in the LoVIC study diagnosed with low VWF after menarche were included in the analysis. Enrollment of female patients presenting during pregnancy was deferred until they had reached 6 months postpartum. Data were obtained between October 2014 and October 2017, with source of referral determined from review of medical records with the participant’s consent.
Bleeding score
At enrollment, all participants completed a bleeding questionnaire from which both the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH BAT) and Condensed Molecular and Clinical Markers for the Diagnosis and Management of type 1 VWD (Condensed MCMDM-1 VWD) scores could be derived. This questionnaire was administered by a physician specialized in thrombosis and hemostasis and included only symptoms prior to the diagnosis of low VWF. For this reason, all female enrollees diagnosed premenarche were excluded from this analysis. A positive ISTH BAT score was defined as ≥6 and Condensed MCMDM-1 VWD score ≥4, in keeping with the published literature.17,18 The outline of each score for the gynecological domains is provided in supplemental Table 1.
Laboratory assessments
All historic and study assays were performed at a single center, the National Coagulation Laboratory, St. James’s Hospital. Laboratory investigations were performed as outlined previously.16 Following collection of venous blood into 3.2% sodium citrate, platelet-poor plasma was generated by double centrifugation at 3000g for 10 minutes. Samples were stored at −70°C until analysis. Plasma VWF:Ag levels were measured using a latex particle-enhanced immunoturbidimetric assay (HemosIL VWF antigen assay; Instrumentation Laboratories, IL, Milan, Italy) on an automated coagulometer (ACL Top 700; Instrumentation Laboratories, Milan, Italy). Plasma VWF:CB levels were assessed using a commercial enzyme-linked immunosorbent assay (ELISA) (Technoclone; Techonozym, Vienna, Austria). Plasma VWF:RCo levels were measured using a standard platelet agglutination technique on a Sysmex CS2100i Analyzer (Siemens Healthcare, Marburg, Germany). Plasma coagulation factor VIII levels were determined using standard 1-stage clotting assays (ACLTop700; Instrumentation Laboratory, Milan, Italy). Ferritin levels were measured using a commercial immunoassay (Advia Centaur Ferritin assay on an Advia Centaur XP; Siemens Healthcare, Marburg, Germany).
Statistical methods
Laboratory and clinical data were expressed using mean or median depending on distribution and continuous variables such as ISTH BAT and Condensed MCMDM-1 VWD bleeding scores expressed as medians. Descriptive statistics are presented as frequencies and percentages. The Mann-Whitney U test was used to test the differences in bleeding scores between groups. The Wilcoxon matched pairs signed rank test was used to test the differences between paired nonparametric data such as bleeding scores. P < .05 was considered statistically significant. Statistical analyses were performed using Prism 7 (for Mac OSX, version 7.0c; La Jolla, CA).
Results
One hundred twenty adult women with a registered diagnosis of low VWF who had been recruited through the LoVIC study16 were investigated. The baseline demographics of this subgroup are presented in Table 1. Median age at time of first diagnosis of low VWF was 33 years (range, 14-66 years); median age at enrollment was 38.4 years (range, 18-72 years). These data are very similar to the entire Irish national Low VWF database (which contains a total of 216 female patients registered with low VWF with a median age of 39 years). In keeping with previous reports, blood group O women were significantly overrepresented (89.2% vs 55% of the general Irish population). In addition, despite the moderate reduction in plasma VWF levels in these patients, the majority of women demonstrated significantly elevated ISTH (79.2% women with scores ≥6; median score, 8; range, 0-24) and MCMDM-1 VWD (73.3% women with scores ≥4; median score, 5; range, −2 to 18) bleeding scores (Table 1).
Table 1.
Patient characteristics of the women included in the cohort
| LoVIC | |
|---|---|
| Diagnosis, n | |
| Low VWF | 120 |
| Age, median (range), y | |
| At diagnosis | 33 (14.0-66.0) |
| At enrollment | 38.4 (18.0-72.0) |
| Blood group, n (%) | |
| O | 107 (89.2) |
| A | 10 (8.3) |
| B | 2 (1.6) |
| AB | 1 (0.8) |
| Plasma VWF levels, median (range), IU/dL | |
| Enrollment levels | |
| VWF:Ag | 59 (36-116) |
| VWF:RCo | 46 (30-108) |
| VWF:CB | 49 (30-100) |
| FVIII:C | 75 (36-75) |
| Lowest levels | |
| VWF:Ag | 49 (33-72) |
| VWF:RCo | 39 (30-54) |
| VWF:CB | 42 (30-91) |
| FVIII:C | 69 (29-128) |
| VWF:RCo/VWF:Ag | 0.79 (0.51-1.13) |
| VWF:CB/VWF:Ag | 0.84 (0.57-1.34) |
| Bleeding scores | |
| ISTH BAT score | |
| Median score (range) | 8 (0-24) |
| Positive score ≥6, n (%) | 95 (79.2) |
| Condensed MCMDM-1 VWD score | |
| Median score (range) | 5 (−2 to 18) |
| Positive score ≥4, n ( %) | 88 (73.3) |
Menorrhagia is the most frequent bleeding symptom in women with low VWF levels
To investigate the nature of bleeding complications in women with low VWF, individual domain scores within the ISTH BAT and MCMDM-1 VWD score were examined. Overall, 89% of the females enrolled reported symptoms of menorrhagia (Table 2). Furthermore, 76% of the total cohort reported changing pads/tampons more frequently than 2 hourly and 57% had bleeding lasting >7 days.
Table 2.
Reported menorrhagia and treatment received prior to diagnosis with low VWF
| Menorrhagia | LoVIC, n (% of all female enrollees) |
|---|---|
| Total cohort | 120 |
| Self-reported menorrhagia | 107/120 (89.2) |
| Symptoms | |
| Changing pads/tampons >2 hourly | 92 (76.6) |
| Bleeding >7 d | 69 (57.5) |
| Clots and flooding | 104 (86.6) |
| Medical consultation | |
| Consultation | 73 (60.8) |
| Iron therapy | 39 (32.5) |
| Antifibrinolytic | 23 (19.2) |
| Hormonal therapy | 47 (39.2) |
| Combined hormonal and antifibrinolytics | 8 (6.6) |
| Intrauterine levonorgestrel-releasing device | 20 (16.7) |
| Dilatation and curettage | 23 (19.2) |
| Endometrial ablation | 5 (4.2) |
| Hysterectomy | 9 (7.5) |
| Treatment with desmopressin | 1 (0.8) |
| Treatment with plasma/platelets/factor concentrate | 0 |
| Red blood cell transfusion | 2 (1.7) |
| Hospital admission and emergency treatment | 2 (1.7) |
| Duration of symptoms | |
| Symptoms since menarche | 84 (70.0) |
| >2 d off school/work per year | 47 (39.2) |
| Menorrhagia >12 mo | 21 (17.5) |
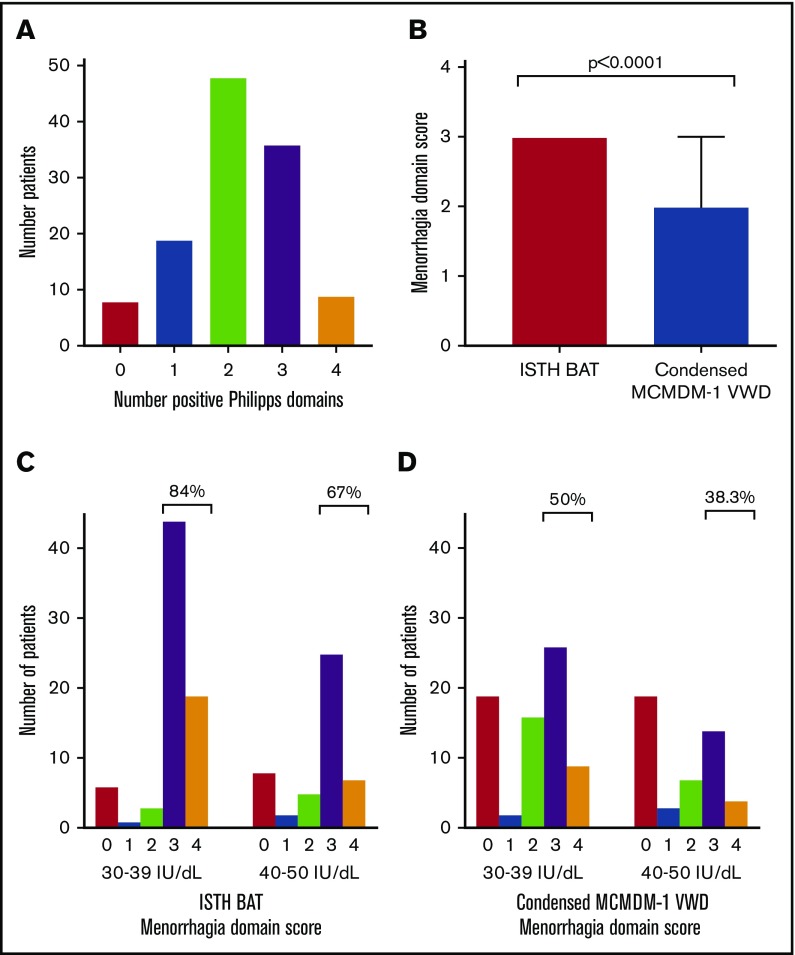
In addition to BAT, other scoring tools including the Philipps score19 have been developed to identify women with HMB who may warrant hemostatic investigations. Using this tool, 93.3% (n = 112) of our cohort had ≥1 positive domains, (Figure 1A). Nevertheless, in spite of these symptoms, only 60% (n = 73) of the women with low VWF levels studied had presented for medical consultation regarding HMB prior to diagnosis. Interestingly, there was no evidence that the diagnosis of low VWF was expedited in women who presented with HMB (34.2 years at diagnosis for women who sought medical consultation vs 33.4 years for women who did not attend; P = .7).
Figure 1.
Philipps score and menorrhagia bleeding scores in women with low VWF. (A) Bar chart of the number of women with positive responses to the 4 domains included in the Philipps tool. (B) Comparison of the ISTH BAT and Condensed MCMDM-1 VWD menorrhagia-domain score in women with low VWF using identical clinical information (median 3 vs 2, P < .0001, median and interquartile range [IQR], indicated for Condensed MCMDM-1 VWD; for the ISTH BAT both the median and IQR are equal to 3). (C-D) Bar chart of menorrhagia-domain bleeding scores by lowest recorded plasma VWF levels. When examined by lowest recorded plasma VWF levels, 84% of those female enrollees with lowest levels (30-39 IU/dL) had ISTH BAT menorrhagia-domain scores ≥3 in comparison with 67% of patients with lowest plasma VWF level (40-50 IU/dL) (C). A similar pattern was seen using the Condensed MCMDM-1 VWD score (D).
Examination of patient case records demonstrated that only 25.8% (n = 31) of participants were directly referred from their primary care physician due to bleeding and/or abnormal laboratory tests. In contrast, 37.5% of women were referred on the basis of a positive family history of a bleeding disorder. Interestingly, 36.6% of women had been reviewed by both a primary care physician and at least 1 hospital specialist (gynecology, otolaryngology, surgery, general hematology) prior to their initial referral. Moreover, of the 25 women seen by gynecology, 11 of 25 women underwent an endometrial ablation and/or hysterectomy prior to their National Coagulation Centre assessment. All together, these findings demonstrate that HMB is common in women with low VWF levels. Moreover, despite their bleeding phenotype, there is clearly a significant delay before many female patients with HMB present for medical consultation.
HMB is associated with significant morbidity in women with low VWF
Using the ISTH BAT menorrhagia bleeding score domain, additional information regarding the duration of menorrhagia and impact on daily living was assessed. Overall, 70% of women described heavy periods since menarche, with an additional 17.5% reporting menorrhagia for over 12 months (Table 2). Furthermore, 39.2% of women reported missing >2 days off work or school per year due to menorrhagia. Of the 73 women with low VWF who sought medical consultation for HMB prior to being diagnosed with low VWF, 49 (67.1%) were prescribed hormonal therapy, either on its own (combined oral contraceptive pill or hormonal releasing intrauterine device) or in combination with antifibrinolytic agents. Nevertheless, despite these interventions, many of the patients (34.2%) subsequently still required surgical interventions (including dilatation and curettage, endometrial ablation, or hysterectomy). Even following formal diagnosis with low VWF, HMB remained a significant ongoing management challenge necessitating treatment with tranexamic acid (n = 42, 41.6%), hormonal therapy (n = 25, 24.8%), and intrauterine devices (n = 15, 14.9%). Furthermore, 33 women (32.7%) required gynecological review due to ongoing HMB after their initial registration with low VWF. Of these, 11 women proceeded to dilatation and curettage and 4 underwent endometrial ablation/hysterectomy. Overall, although 58 women of the 120 women with low VWF studied attended for gynecology review at some stage (25 pre- and 33 post diagnosis), a significant contributing gynecological pathology (fibroids) for HMB was identified in only 1 patient.
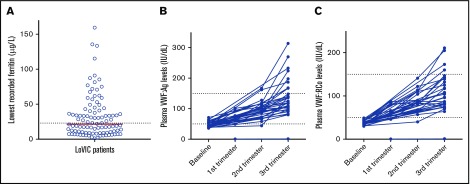
Previous studies have reported lower quality of life (QoL) scores and iron deficiency in women with reduced plasma VWF levels.20 To determine the rate of iron deficiency in our cohort of females with low VWF, previous hemoglobin and ferritin levels were reviewed. Of the total cohort of 120 women, 310 plasma ferritin levels on 103 individuals were identified. Importantly, all of these tests were performed following formal registration with a bleeding disorder. Nevertheless, reduced ferritin levels were observed in n = 55 (45.8%) of those studied (Figure 2A). Furthermore, overt iron-deficiency anemia was seen in 26 patients (21.7%). Collectively, these data highlight the significant morbidity associated with HMB in women with low VWF levels.
Figure 2.
Ferritin levels in women with low VWF and pregnancy related changes in plasma VWF levels. (A) Scatter dot plot of lowest recorded ferritin (n = 103) with the median (21.1 μg/L) indicated by the red bar and lower limit of the normal reference range (23.0-393 μg/L) by the dotted line. (B-C) Plasma VWF:Ag levels (B) and VWF:RCo levels (C) at baseline and during pregnancy in a subgroup of 32 women (38 pregnancies). Normal reference range indicated by dotted lines.
ISTH BAT vs Condensed MCMDM-1 VWD scores for HMB evaluation in women with low VWF
The ISTH BAT and Condensed MCMDM-1 VWD scores have both been validated for the objective assessment of bleeding in patients with VWD.17,18 Using both bleeding scores, we observed that menorrhagia was the most frequently reported bleeding symptom in women with low VWF (88.3% of patients scoring ≥1 using the ISTH BAT and 67.5% of patients using the Condensed MCMDM-1 VWD score). Importantly however, although the questionnaire (from which both the ISTH and Condensed MCMDM-1 VWD scores were derived) was administered by the same physician on the same day, we observed important differences between the scores. In particular, menorrhagia domain scores were significantly higher when assessed by ISTH as opposed to Condensed MCMDM-1 VWD BAT (Figure 1B). For example, using, the Condensed MCMDM-1 VWD score only 53 of 120 women (44.2%) had menorrhagia-domain scores of ≥3 as compared with 95 of 120 (79.2%) when assessed using the ISTH BAT. This difference relates to the fact that a positive score in the menorrhagia domain of the Condensed MCMDM-1 VWD score requires that women must have undergone medical evaluation and treatment. In contrast, the ISTH BAT incorporates patient reporting of HMB including time off school/work, duration of symptoms, presence of clots or flooding, and need for frequent pad changes. Because 47 of 120 (40%) of our cohort of women with low VWF had not sought medical review for their menorrhagia, this resulted in a score of 0 in the menorrhagia domain of the Condensed MCMDM-1 VWD (despite the fact that 32 of these 47 patients reported heavy periods since menarche, resulting in a median ISTH BAT menorrhagia-domain score of 3). This discrepancy between the BATs limits the use of the Condensed MCMDM-1 VWD score in this context, and further suggests that the ISTH BAT is more sensitive for the assessment of HMB in female patients.
Impact of plasma VWF levels and concomitant hemostatic defects on menorrhagia
Previous studies have demonstrated that in patients with VWD, bleeding scores and menorrhagia inversely correlate with plasma VWF levels.21 In contrast, however, we observed no significant difference in ISTH BAT or Condensed MCMDM-1 VWD menorrhagia scores for female patients with VWF levels in the 30 to 39 IU/dL range compared with those with lowest VWF levels in the range 40 to 50 IU/dL (Figure 1C-D). Interestingly, however, a trend toward more HMB in women with levels in the 30 to 39 IU/dL range was seen using both bleeding scores. For example, an ISTH menorrhagia-domain score ≥3 was found in 84% of women with plasma VWF levels 30 to 39 IU/dL in comparison with 67% of women with baseline plasma VWF levels of 40 to 50 IU/dL (Figure 1C). Similarly, using the Condensed MCMDM-1 score, 50% of the women with levels 30 to 39 IU/dL had a menorrhagia-domain score ≥3 in comparison with only 38.3% of those with baseline plasma VWF levels in the 40 to 50 IU/dL range (Figure 1D).
To investigate whether additional concurrent bleeding disorders may contribute to the gynecological bleeding in our low VWF cohort, platelet aggregation studies and plasma clotting factor activity levels (FII, FV, FVII, FVIII, FIX, FX, FXI, FXIII) were investigated for each woman enrolled. Mild reductions in clotting factor levels were observed in only 7 (5.8%) patients. Of these, 4 patients had reductions in plasma FVIII:C levels (28, 28, 36, and 39 IU/dL; normal range, 60-136 IU/dL), whereas the other 3 patients had isolated mild reductions in FX:C (0.61 IU/dL; normal range, 78-142 IU/dL), FXI:C (0.58 IU/dL; normal range, 72-152 IU/dL), and FXIII:C (0.48 IU/dL; normal range, 73-160 IU/dL), respectively. Platelet aggregation studies demonstrated subtle abnormalities in a further 3 patients (2.5%). Importantly, there was no significant difference in gynecological bleeding scores for patients with low VWF and concomitant hemostatic abnormalities (n = 10) compared with women with low VWF patients (n = 110) who had no other abnormalities (median ISTH BAT scores 8.5 vs 9; P = .8). All together, these data demonstrate that in most women with low VWF, the gynecological bleeding phenotype cannot be explained by the presence of detectable concomitant bleeding disorders.
PPH is increased in women with low VWF levels
Within the cohort examined, 74 women had at least 1 pregnancy prior to their diagnosis with low VWF. One hundred eighty-one successful deliveries were reported, with a mean live birth rate of 2.5 per woman. Surprisingly, given the increase in plasma VWF levels associated with pregnancy, 63.5% of parous women (47 of 74) reported excess bleeding at time of delivery (Table 3). In contrast to the differences between the ISTH BAT or Condensed MCMDM-1 VWD scores in HMB assessment in women with low VWF, we observed no significant differences in their respective sensitivities to PPH. As both scores involve self-reported bleeding questionnaires, we considered whether recall bias might be contributing to the apparent high PPH rates reported. However, 21.6% of women (16 of 74) had PPH-domain bleeding scores ≥3 using the ISTH BAT score (indicating that transfusion, critical care, radiological or surgical intervention was required) (Table 3). Overall, 10.8% of patients required assessment in the operating room with an examination under anesthesia, and 9.5% of women with low VWF required postpartum blood transfusion. Moreover, PPH resulted in a delay in discharge from hospital or a need for readmission in 25.7% of the parous women.
Table 3.
Self-reported PPH and treatment received prior to diagnosis with low VWF in subgroup of parous women (n = 74)
| PPH | LoVIC, n (% of all female enrollees) |
|---|---|
| Parous female enrollees | 74/120 (61.7) |
| No. of successful pregnancies | 181 |
| Mean deliveries per parous female enrollee | 2.5 |
| Self-reported PPH, n (% parous female enrollees) | 47/74 (63.5) |
| First 24 h only | 22 (29.7) |
| 24 h to 6 wk only | 11 (14.9) |
| Both | 14 (18.9) |
| Delayed discharge/readmission | 19 (25.7) |
| Medical treatment, n (% parous female enrollees) | |
| Consultation/oxytocin IV | 40 (54.0) |
| Iron therapy | 15 (20.3) |
| Antifibrinolytic therapy | 3 (4.1) |
| Additional uterotonics | 2 (2.7) |
| Examination under anesthesia | 8 (10.8) |
| Uterine tamponade | 1 (1.4) |
| Treatment with desmopressin | 1 (1.4) |
| Treatment with plasma/platelets/factor concentrate | 0 |
| Red blood cell transfusion | 7 (9.5) |
| ICU admission/surgical intervention | 1 (1.4) |
| Lochia, n (% parous female enrollees) | |
| Lochia >6 wk | 19 (25.7) |
| Changing pads/tampons >2 hourly | 31 (41.9) |
ICU, intensive care unit.
Following registration with low VWF, 32 of the women enrolled in the LoVIC study subsequently underwent 38 pregnancies. Critically, in all of these women with low VWF, plasma VWF:Ag and VWF:RCo levels had corrected to within or above our normal nonpregnant plasma VWF normal range by the third trimester (Figure 2B-C). Consequently, no additional hemostatic therapies were administered prior to delivery. Interestingly, however, a threefold variation in third-trimester VWF levels was observed between different individual women (median VWF:Ag, 123 IU/dL; range, 80-270 IU/dL [median VWF:RCo, 104 IU/dL; range, 64-211 IU/dL]). Excess bleeding was recorded in 8 of 38 deliveries (5 at time of delivery and 3 women with prolonged lochia >6 weeks).
Discussion
Although menorrhagia represents the most frequent bleeding symptom in women with VWD,22,23 the incidence and prevalence of HMB in women registered with low VWF levels has not been previously defined. In this study, we demonstrate for the first time that HMB is also a prevalent and important clinical issue in women with low VWF levels. Interestingly, despite the fact that these patients have relatively moderate reductions in plasma VWF levels (range, 30-50 IU/dL), we found that almost 90% had significant self-reported menorrhagia. Our findings also highlight the direct clinical and socioeconomic importance of HMB in women with low VWF. For example, 40% of women with low VWF reported missing >2 days off work or school per year due to menorrhagia. Moreover, by the time that these women with low VWF reported for clinical review, >50% required iron-replacement therapy. Following diagnosis, 45.8% of women tested were iron deficient with iron-deficiency anemia in 21.7%. In addition, despite iron-replacement therapy, we observed evidence of recurrent iron deficiency in 27 patients (median ferritin testing 3.5 years apart). Although 70% of women in our cohort described menorrhagia dating back to their menarche, surprisingly only 60% had presented for medical consultation regarding their HMB. All together, these data have significant public health ramifications given that an estimated ∼0.3% of the general female population will have low VWF levels in the 30 to 50 IU/dL range.12 Consequently, for example, this diagnosis may apply to an estimated 7250 women in Ireland and as many as 0.5 million women in the United States alone.
Although we observed that >30% of women with low VWF levels required surgical intervention for HMB (including dilatation and curettage, and endometrial ablation), only 9% had required hysterectomy prior to their enrollment in the LoVIC study. As the LoVIC study enrolled only those with plasma VWF levels in the 30 to 50 IU/dL range, it is not possible to directly compare with a locally matched VWD population. However, among those women with type 1 VWD enrolled in the Dutch WIN study (n = 242), 24% of women had undergone a hysterectomy.3 This difference likely relates in large part to the differences in plasma VWF levels between the cohorts. In addition, the median age of women in LoVIC (33 years) was lower than in WIN (46 years).3 Finally, in the LoVIC study, only bleeding symptoms and surgical procedures conducted prior to the patient’s formal diagnosis with low VWF were included as opposed to up to the time of enrollment in the WIN study. Clearly, in such retrospective studies, all bleeding data derived from the use of self-reported questionnaires is susceptible to inherent risk of recall bias. Nonetheless, additional data collected on our cohort of women with low VWF following initial formal diagnosis emphasizes that HMB continues to pose significant ongoing management challenges, even in the setting of a comprehensive care center for patients with bleeding disorders.
In terms of objectively assessing bleeding in patients with VWD, consensus guidelines recommend the use of BAT scores. Although the utility of several bleeding questionnaires has been validated in patients with VWD, no previous head-to-head comparative studies have been performed so it remains unclear whether any specific BAT is preferable. Our data clearly demonstrate that in terms of assessing HMB in women with low VWF levels, important differences exist between the ISTH BAT and the Condensed MCMDM-1 VWD scores. In particular, using the ISTH BAT, 95 patients (79.2%) recorded menorrhagia-domain score ≥3. However, using the Condensed MCMDM-1 VWD score (administered by the same physician on the same day), only 53 (44.1%) of the same women had menorrhagia-related scores ≥3. This difference is explained by the fact that women must have undergone a medical evaluation in order to gain a positive score in the menorrhagia domain of the latter BAT. Thus, given that 40% of our women with low VWF had never sought medical review for their menorrhagia, it is perhaps unsurprising that the ISTH BAT score was significantly more sensitive for assessment of HMB in women with low VWF levels. Collectively, on the basis of our data, we propose that the ISTH BAT score should be considered the preferred BAT in the diagnosis of low VWF levels and VWD.
Recent international consensus clinical guidelines have proposed different plasma VWF threshold levels for assigning a diagnosis of low VWF (either 30-40 IU/dL or 30-50 IU/dL).12,15 In this study, we observed a nonsignificant trend toward increased HMB in women (84% with ISTH BAT menorrhagia-domain scores ≥3) with low VWF levels in the 30 to 40 IU/dL range. Importantly, however, 67% of women with low VWF levels in the 40 to 50 IU/dL range also had significantly elevated menorrhagia-domain scores. Given the minimal reductions involved in plasma VWF in this latter cohort, it is not clear whether the HMB described is simply attributable to impaired primary hemostasis, or whether other functions of VWF may contribute to the significant gynecological bleeding phenotype observed in women with low VWF levels. Recent studies have shown that VWF has roles in modulating both inflammation and angiogenesis.24,25 In particular, recent studies of blood outgrowth endothelial cells have suggested that reductions in VWF may impact upon angiogenesis in vitro.26-28 Further studies will be required to investigate the in vivo significance of these findings and their impact on the phenotype observed.
In addition to the clinical importance of HMB in women with low VWF, our data further highlight the prevalence of PPH in this cohort of patients. This increase in PPH is surprising given the fact that plasma VWF levels typically increase during the course of normal pregnancy. Consequently, plasma VWF concentrations would be expected to correct into the normal range (50-150 IU/dL) for most female patients with low VWF levels during the course of their pregnancy. Nonetheless, 21.6% women (16 of 74) recorded an ISTH BAT PPH-domain score of ≥3, indicating the need for either examination under anesthesia, tamponade, transfusion, or critical care. These intriguing data clearly suggest that some women with low VWF may still be at risk of PPH despite the pregnancy-related increase in plasma VWF levels. Utilization of normal plasma VWF reference ranges established on nonpregnant individuals fails to adjust for the physiological increase in plasma VWF levels. Therefore, although plasma VWF levels may increase in women with low VWF, they may still be lower than expected for gestation. All together, our findings together with previous reports29-32 suggest that treating physicians may need to consider maintaining higher plasma VWF levels peripartum. Further studies will be needed to elucidate the mechanisms underlying PPH in women with low VWF levels and to define optimal strategies for the clinical management of these patients.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by an Irish Health Research Board Health Research Award (HRA-POR-2014-529) (J.S.O.) and a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O.).
Footnotes
Presented in abstract form as an oral presentation at the Congress of the International Society on Thrombosis and Haemostasis, Berlin, Germany, 8 July 2017.
Authorship
Contribution: P.D.J. designed the integrated questionnaire; M.N., M.L., K.R., B.W., N.M.O., and J.S.O. conducted the research; M.L., S.A., N.D., J.M.O., and J.S.O. analyzed the data; and all authors (M.L., S.A., N.D., M.N., M.B., B.W., K.R., N.M.O., J.M.O., J.D.P., P.D.J., and J.S.O.) were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: M.L. has served on a speaker’s bureau for Shire and received research funding from Baxter. J.S.O. has served on the speaker’s bureaus for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma Shire, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Shire, and Pfizer; and has also received research grant funding awards from Baxter, Bayer, Pfizer, Shire, and Novo Nordisk. P.D.J. has received research funding from CSL Behring, Bayer, and Shire. The remaining authors declare no competing financial interests.
Correspondence: Michelle Lavin, Irish Centre for Vascular Biology, Royal College of Surgeons Ireland, Ardilaun House, 111 St. Stephen’s Green, Dublin 2, Ireland; e-mail: michellelavin@rcsi.ie.
References
- 1.von Willebrand E. Hereditar psuedohemofili. Fin Lakaresallsk Handl. 1926;67:7-112. [Google Scholar]
- 2.Committee on Adolescent Health Care; Committee on Gynecologic Practice. Committee opinion no.580: von Willebrand disease in women. Obstet Gynecol. 2013;122(6):1368-1373. [DOI] [PubMed] [Google Scholar]
- 3.De Wee EM, Knol HM, Mauser-Bunschoten EP, et al. ; WiN Study Group. Gynaecological and obstetric bleeding in moderate and severe von Willebrand disease. Thromb Haemost. 2011;106(5):885-892. [DOI] [PubMed] [Google Scholar]
- 4.Kadir RA, Economides DL, Sabin CA, Pollard D, Lee CA. Assessment of menstrual blood loss and gynaecological problems in patients with inherited bleeding disorders. Haemophilia. 1999;5(1):40-48. [DOI] [PubMed] [Google Scholar]
- 5.Kouides PA, Phatak PD, Burkart P, et al. . Gynaecological and obstetrical morbidity in women with type I von Willebrand disease: results of a patient survey. Haemophilia. 2000;6(6):643-648. [DOI] [PubMed] [Google Scholar]
- 6.Ragni MV, Bontempo FA, Hassett AC. von Willebrand disease and bleeding in women. Haemophilia. 1999;5(5):313-317. [DOI] [PubMed] [Google Scholar]
- 7.Rae C, Furlong W, Horsman J, et al. . Bleeding disorders, menorrhagia and iron deficiency: impacts on health-related quality of life. Haemophilia. 2013;19(3):385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar M, Lee CA, Sabin CA, Economides DL, Kadir RA. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111(7):734-740. [DOI] [PubMed] [Google Scholar]
- 9.James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5(6):1165-1169. [DOI] [PubMed] [Google Scholar]
- 10.Malec LM, Moore CG, Yabes J, Li J, Ragni MV. Postpartum haemorrhage in women with von Willebrand disease: an observational study of the Pennsylvania Health Care Cost Containment Council (PHC4) database. Haemophilia. 2015;21(5):e442-e445. [DOI] [PubMed] [Google Scholar]
- 11.Ramsahoye BH, Davies SV, Dasani H, Pearson JF. Obstetric management in von Willebrand’s disease: a report of 24 pregnancies and a reviews of the literature. Haemophilia. 1995;1(2):140-144. [DOI] [PubMed] [Google Scholar]
- 12.Nichols WL, Hultin MB, James AH, et al. . von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232. [DOI] [PubMed] [Google Scholar]
- 13.Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. Pregnancy in women with von Willebrand’s disease or factor XI deficiency. BJOG. 1998;105(3):314-321. [DOI] [PubMed] [Google Scholar]
- 14.Mannucci PM. How I treat patients with von Willebrand disease. Blood. 2001;97(7):1915-1919. [DOI] [PubMed] [Google Scholar]
- 15.Laffan MA, Lester W, O’Donnell JS, et al. . The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavin M, Aguila S, Schneppenheim S, et al. . Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbatarny M, Mollah S, Grabell J, et al. ; Zimmerman Program Investigators. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman M, Mundell G, Grabell J, et al. . Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6(12):2062-2066. [DOI] [PubMed] [Google Scholar]
- 19.Philipp CS, Faiz A, Dowling NF, et al. . Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198(2):163.e1-163.e8. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Deforest M, Grabell J, Hopman W, James P. Relative contributions of bleeding scores and iron status on health-related quality of life in von Willebrand disease: a cross-sectional study. Haemophilia. 2017;23(1):115-121. [DOI] [PubMed] [Google Scholar]
- 21.Kujovich JL. von Willebrand’s disease and menorrhagia: prevalence, diagnosis, and management. Am J Hematol. 2005;79(3):220-228. [DOI] [PubMed] [Google Scholar]
- 22.de Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. ; WiN Study Group. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683-692. [DOI] [PubMed] [Google Scholar]
- 23.James AH, Kouides PA, Abdul-Kadir R, et al. . Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):124-134. [DOI] [PubMed] [Google Scholar]
- 24.Starke RD, Ferraro F, Paschalaki KE, et al. . Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117(3):1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denis CV, André P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98(7):4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvam S, James P. Angiodysplasia in von Willebrand disease: understanding the clinical and basic science. Semin Thromb Hemost. 2017;43(6):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starke RD, Paschalaki KE, Dyer CEF, et al. . Cellular and molecular basis of von Willebrand disease: studies on blood outgrowth endothelial cells. Blood. 2013;121(14):2773-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groeneveld DJ, van Bekkum T, Dirven RJ, et al. . Angiogenic characteristics of blood outgrowth endothelial cells from patients with von Willebrand disease. J Thromb Haemost. 2015;13(10):1854-1866. [DOI] [PubMed] [Google Scholar]
- 29.James AH, Konkle BA, Kouides P, et al. . Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21(1):81-87. [DOI] [PubMed] [Google Scholar]
- 30.Kouides PA. Preventing postpartum haemorrhage–when guidelines fall short. Haemophilia. 2015;21(4):502-504. [DOI] [PubMed] [Google Scholar]
- 31.Ragni MV. Blood volume-based von Willebrand factor to prevent postpartum hemorrhage in von Willebrand disease. Blood Adv. 2017;1(11):703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragni MV, Machin N, James AH, et al. . Feasibility of the von Willebrand disease PREVENT trial. Thromb Res. 2017;156:8-13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.