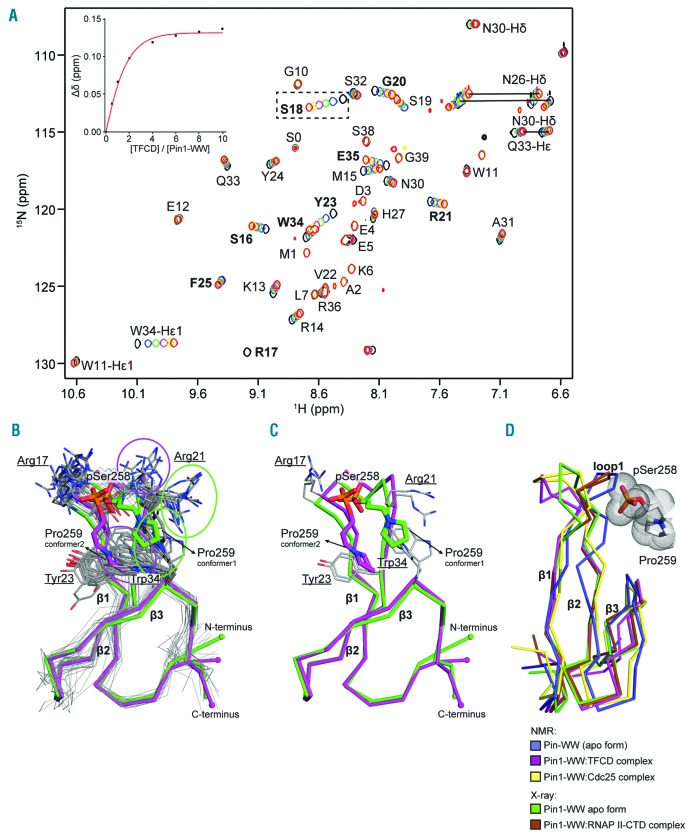
Figure 3.
Nuclear magnetic resonance (NMR) spectroscopy shows interaction between twenty-amino acid cytoplasmic domain (TFCD) and Pin1 requires phosphorylation of Ser258 and trans-configuration of the pSer258-Pro259 peptide bond in the TFCD. (A) Superimposition of the assigned 1H/15N HSOC spectra of the Pin1 WW-domain with double phosphorylated TFCD (pSer253/pSer258) showing the chemical shift changes upon increasing amount of peptide to a 10× molar excess. A non-linear regression fit of the Ser18 (peak shift shown in black dotted box) was used to calculate the binding constant of the complex. (B) Bundle of 20 NMR conformers sampling into two major conformers. Sidechains of Pro259 (TFCD), Arg21 and Trp34 (Pin1 WW-domain) are indicated with circles. (C) Representative models of the two lowest-energy conformations from (B) are shown in green and magenta. The polypeptide backbones are shown as ribbons. Pin1 WW-domain residues are underlined. (D) Contact of the TFCD pSer258-Pro259 motif with the Pin1 WW-domain loop1 and comparison of NMR and X-ray structures.