Visual Abstract
Key Words: biomaterials, device, hydrogel, pericardial delivery
Abbreviations and Acronyms: CVD, cardiovascular disease; miRNA, micro-ribonucleic acid; PEG, polyethylene glycol
Highlights
-
•
The pericardial space is an unexploited anatomic location for hydrogel delivery.
-
•
Hydrogels can be delivered to the pericardial space in a localized, minimally invasive manner, without detectable hemodynamic effects.
-
•
Pericardial hydrogel delivery is a new strategy to direct therapeutics to the heart with reduced systemic delivery and off-target effects.
Summary
Biomaterials are a new treatment strategy for cardiovascular diseases but are difficult to deliver to the heart in a safe, precise, and translatable way. We developed a method to deliver hydrogels to the epicardium through the pericardial space. Our device creates a temporary compartment for hydrogel delivery and gelation using anatomic structures. The method minimizes risk to patients from embolization, thrombotic occlusion, and arrhythmia. In pigs there were no clinically relevant acute or subacute adverse effects from pericardial hydrogel delivery, making this a translatable strategy to deliver biomaterials to the heart.
Despite pharmacological and technologic advances, cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in the United States, costing $215.6 billion per year (1). More patients are surviving, but with heart failure, arrhythmias, and poor quality of life. Micro-ribonucleic acid (miRNA), gene therapy, stem cells, cytokines, and other biologics are new treatments that have shown promise in preclinical and early phase clinical trials 2, 3, 4. Many of these therapies require focused delivery of the therapeutic to the heart, or even localization to particular anatomic areas, such as the peri-infarction region. Dilution of these therapeutics by systemic administration increases cost and risks off-target effects. For example, poor retention of stem cells in the heart is thought to limit efficacy in clinical trials 5, 6, 7. The proangiogenic cytokine vascular endothelial growth factor encourages neoangiogenesis and cardiac regeneration (8) but can also accelerate tumor metastasis (9). Efficient, targeted, and temporally appropriate delivery of therapeutics to the heart are keys to their successful translation into clinical use.
Early phase clinical trials are underway using hydrogels as therapeutic agents for cardiac repair 10, 11, 12. Both solid patches and injectable gel materials are under investigation and may have benefits for different applications. Cardiac patches and solid materials have been tested as structural support for the heart in a clinical trial (13) and therapeutic delivery platforms in numerous preclinical studies (14). Their widespread use is limited by the need for surgical placement. Injectable materials with liquid or gel phases, such as decellularized matrix, alginate, and engineered hydrogels, can provide scaffolds, tactile signals, and structural support for cardiac regeneration and repair 10, 11, 15, 16. Biomaterial gels are particularly suited to deliver stem cells to the heart and retain viable cells at the site of delivery 12, 17. Other materials are in preclinical trial for delivery and sustained release of miRNAs, cytokines, and other therapeutics 4, 18, 19. Whereas biocompatible materials may be beneficial for the treatment of CVD, there are no dedicated delivery methods that are safe and minimally invasive.
There are challenges inherent to delivering biomaterials to the heart. Open heart surgery, although feasible, is less desirable from a cost and patient perspective. Catheter delivery using commercially available single-lumen coronary catheters or Noga XP Cardiac Navigation System (Biosense Webster, Diegem, Belgium) cannot keep material components separate as they travel to the heart and thus cannot control the timing of material interaction and gelation. Premature gelation causes clogging within catheter lumen. Delayed gelation can lead to embolization, stroke, and failure to deliver material to targeted area. Another challenge with biomaterial delivery to the heart is the potential for inducing arrhythmias if the electrical conductivity of the material creates a substrate for a re-entrant circuits as it interdigitates between cardiomyocytes. Therefore the development of material-specific strategies is necessary for the safe, precise, and practical delivery of biomaterial to the heart.
The pericardium is a novel site for therapeutic delivery that has been shown in animal studies to act as a reservoir for drug delivery to the heart 20, 21, 22. The advent of epicardial ablations and external left atrial appendage ligation has demonstrated the feasibility of accessing a “dry” pericardial space for therapeutic purposes 23, 24, 25. Herein we describe a minimally invasive device to deliver biomaterials to the heart by using the pericardial space as a novel anatomic site for biomaterial delivery. Our device uses the existing anatomic structures to form a temporary compartment for gel delivery. Features of the device eliminate the risk of premature gelation and embolization and allow precise placement of biomaterial over the area of interest. Pericardial hydrogel delivery with our device circumvents many of the obstacles to vascular or intracardiac delivery, facilitating rapid translation of biomaterial gels into clinical use.
Methods
Device design
We built a device to deliver biomaterial hydrogels to the heart through the pericardial space in a large animal model (pig). The hydrogel delivery device is constructed from varying durometers of polyether block amid (PEBAX) (Temecula Custom Extrusions, Temecula, California) biocompatible polymeric resin, using custom multilumen tooling, extrusion, and fusing processes. Two internal lumens for biomaterials keep components separated throughout the length of the device. The core is comprised of a super-elastic shape-memory nickel titanium alloy (nitinol) that facilitates fence deployment and retraction. Suction and gel ports were cut in the desired location using precision skiving. The sheath is comprised of a laminated composite shaft with an imbedded coil. Key locations of the device and sheath (distal tip, fence apparatus) are fitted with radiopaque markers to enable visualization with fluoroscopy. The device is constructed in a cleanroom and sterilized using ethylene oxide before survival procedures.
Hydrogel gel design and delivery
Polyethylene glycol (PEG) hydrogels were based on 4-arm PEG macromer (20 kDa, Laysan Bio, Arab, Alabama) with maleimides at each terminus cross-linked with dithiothreitol. This platform provides structurally defined hydrogels with stoichiometric incorporation of ligands and improved cross-linking efficiency (18). Hydrogel components (macromer, cross-linker) were delivered into the pericardial space in cadaver and live pigs (n = 9) using the delivery device. The hydrogel components and cross-linker were delivered through separate lumens into the fenced area in the pericardial space for in situ mixing and cross-linking. Hydrogel components were adjusted to yield a 5-ml, 4.0% wt/vol PEG hydrogel. For nonsurvival studies, gel was labeled with radiopaque contrast agent iohexol (Omnipaque, GE Healthcare, Princeton, New Jersey) and in others trypan blue (Sigma, St. Louis, Missouri).
Large animal model
Farm pigs (n = 9; 45 to 55 kg) were obtained from a commercial supplier and raised on swine feed. On the procedure day, animals were sedated with intramuscular telazol (4.4 mg/kg) and xylazine (0.5 mg/kg) and maintained on inhaled isoflurane (2% to 4%). Animals were intubated, ventilated, and continuously monitored. We accessed the pericardial space through a fluoroscopically guided percutaneous subxiphoid approach using 12-cm 21-gauge micropuncture needle. Intrapericardial location was confirmed by contrast injection and wire confinement to the outer cardiac silhouette. Arterial and venous pressures were monitored using a Swan-Ganz and pigtail catheter, respectively, connected to a MacLab Hemodynamic Recording System (GE Healthcare).
A flexible 10-F catheter sheath was placed into the pericardial space. The hydrogel delivery device was positioned over the desired anatomic area of the heart and temporarily secured in place by negative suction. PEG macromere and dithiothreitol (cross-linker) were delivered through two distinct lumens and combined within the fenced region. After allowing 5 min for gelation, the fence was retracted from around the hydrogel. Invasive hemodynamics were measured before pericardial instrumentation, immediately after hydrogel delivery device removal, and at time of sacrifice 4 to 6 weeks later. Animals received intrapericardial methylprednisolone, 1 mg/kg, and oral colchicine, 0.6 mg, by mouth twice daily.
Statistical analysis
Statistical analysis of hemodynamic data from different time points was compared using repeated measures one-way analysis of variance using PRISM version 6 (GraphPad, San Diego, California). A priori power analysis was performed using G*Power version 3.0.10 (Dusseldorf, Germany) with the assumption of α 0.05, power 0.6, and an effect size calculated to 1.0.
Results
Hydrogel delivery device design and construction
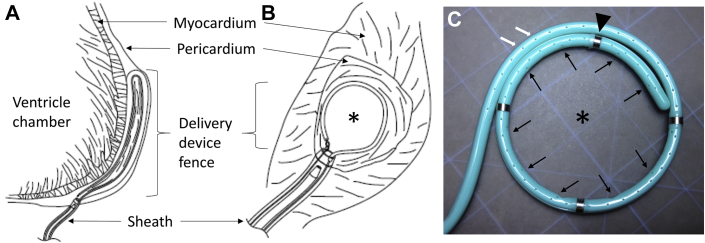
The feasibility and safety of pericardial access in the absence of pericardial effusion (i.e., dry tap) have been shown by other devices, such as the Lariat device (Sentre Heart, Redwood City, California) 23, 24. In this study, we engineered a system consisting of a hydrogel biomaterial delivery device and sheath to access the pericardial space using standard micropuncture technique (Figure 1). Device features include a shape memory core, a deployable and retractable fence that creates the lateral wall of the temporary hydrogel compartment, two separated lumens for gel components, a suction mechanism to temporarily secure the device in place, electrical sensors, and an atraumatic tip (Figure 1).
Figure 1.
Hydrogel Delivery Device Creates a Temporary Compartment for Biomaterial Gel Delivery Within the Pericardial Space
Schematic representation of lateral (A) and frontal view (B) of the device placed over the cardiac epicardium within the pericardial space. The device forms a lateral wall for the gel compartment, whereas the pericardium and epicardium act as a natural roof and floor, respectively (A and B). Once in position, the device is secured in place by gentle suction ports (C, white arrows) to the epicardium as floor, and the pericardium as ceiling. Gel components are delivered through separate lumens and combined only after exiting the device (B and C, asterisk) through ports arrayed around fence (C, black arrows). Electrical sensors (C, arrowhead) allow precise placement over areas of electrical abnormalities, such as infarcts. See Supplemental Video 1.
Deployment and retraction of hydrogel delivery device within the pericardial space in live pigs
Viewing the heart in extreme lateral view, the hydrogel delivery device is placed into the pericardial space using minimally invasive micro-puncture technique. The sheath is positioned over the inferoposterior wall and the delivery device deployed. As the device leaves the sheath, it forms a circular fenced area for biomaterial gel delivery. The pericardium drapes over the device forming a natural roof, while the cardiac epicardium acts as a floor. Hemodynamic measurements were taken using pigtail catheter in left ventricle (arrow) and Swan-Ganz catheter in the pulmonary artery (arrowhead). After allowing sufficient time for hydrogel gelation, the delivery device fence is retracted into the sheath without disruption of gel architecture.
As the delivery device is advanced out of the sheath, it forms a circular fence that physically isolates a 5-cm diameter area of epicardium (Supplemental Video 1). The device forms a lateral border, or fence, while the epicardium acts as a floor, and pericardium acts as a roof for the temporary gel compartment. Gentle suction further seals the compartment and secures the device in place on the moving heart (Supplemental Video 1, Figure 1). Radiopaque markers allow precise placement over desired anatomic area using biplane fluoroscopy. Inside the device hydrogel components are kept within separate internal lumens until delivery through ports arrayed around the circular fenced area (Figure 1C). After gelation, the shape memory core allows retraction of the delivery device without disruption of hydrogel architecture (Supplemental Video 1).
Visualization of hydrogel delivery
Four-arm PEG maleimide macromer was cross-linked with dithiothreitol to form hydrogels (18). This hydrogel system is a flexible platform for therapeutic delivery of stem cells, growth factors, and pancreatic islets in various preclinical models 16, 17, 26. Bench studies showed that gelation occurred within 1 min after combining both components at physiological pH. We tested the delivery device in live pigs (n = 9). One animal was excluded from analysis because of infection unrelated to gel delivery. The delivery procedure was minimally invasive and conducted using standard cardiac catheterization laboratory equipment (Supplemental Figure 1). There were no acute complications with pericardial access, device placement in the pericardial space, or hydrogel deployment. All animals had successful hydrogel placement and solidification. No sustained arrhythmias occurred and there were only occasional premature ventricular contractions during the pericardial access procedure. Retraction of the device respected the hydrogel boundary and did not disrupt the hydrogel by visual inspection (Supplemental Video 1). Pericardial access and delivery procedure took approximately 35 min.
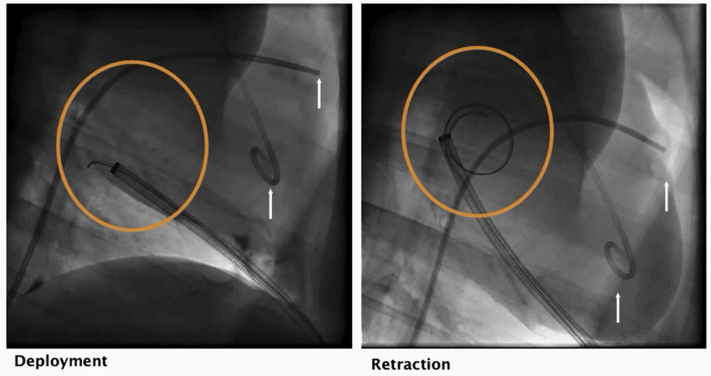
We visualized gelation within the temporary compartment created by the delivery device directly and using fluoroscopy with radiopaque gel (Figures 2A and 2B). Sixty minutes after gel delivery, heart was excised and a well-circumscribed trypan blue gel was present under the pericardium and localized to the inferoposterior wall (Figure 2C). There were no instances of premature gelation within the device in 12 live and cadaver studies. After 4 weeks hearts were excised and examined for gel. On 2 hearts areas of possible gel were observed but could not be confirmed with PEG-directed antibody.
Figure 2.
Successful Hydrogel Delivery to the Pericardial Space Using the Hydrogel Delivery Device Visualized Directly and by Fluoroscopy
(A) The delivery device was successfully placed over the left anterior descending artery in a porcine cadaver. Sternum was removed to allow direct visualization. (B) Contrast-labeled hydrogel delivery by the device (arrow) was visualized in situ by fluoroscopy. (C) In a live animal, trypan blue–labeled gel was delivered with the device to the posterior wall and gelled within the temporary compartment created by the device, epicardium, and pericardium. Sixty minutes after device removal, the gel remained localized over delivery location.
Hemodynamic assessment of epicardial hydrogel delivery
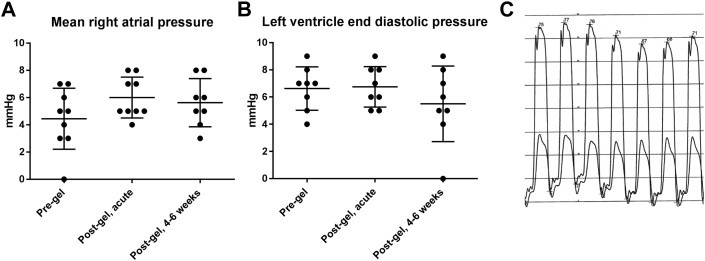
Complications of pericardial access procedures can include tamponade or constriction causing hypotension, tachycardia, and elevated diastolic filling pressures (27). We measured acute and chronic changes in cardiac hemodynamics (4 to 6 weeks) after gel placement. There were no detectable changes in heart rate, blood pressure, right atrial pressure, wedge pressure, or left ventricular end diastolic pressure (Figure 3, Supplemental Table 1). Other hemodynamic features of pericardial construction, such as ventricular discordance, were not detected (Figure 3C). There was no periprocedural mortality. One animal was excluded from analysis because of infection unrelated to pericardial procedure but showed no hemodynamic abnormality.
Figure 3.
Invasive Hemodynamics Were Unchanged After Pericardial Gel Placement
The right atrium is the lowest pressure cardiac chamber with the thinnest wall and most susceptible to compression from increased pericardial pressure. (A and B) Right atrial pressure and left ventricle end-diastolic pressure did not change in pigs in the immediate post-procedure period or after 4 to 6 weeks (n = 8; error bars ± SD). (C) Simultaneous left and right ventricle pressure showed no accentuated respiratory variation and no ventricular interdependence (paper speed 25 mm/s, representative image).
Inflammatory effects of pericardial procedure and gel delivery
Pericarditis is a well-known consequence of pericardial procedures and we evaluated animals for signs of systemic or local inflammation 23, 24, 25. We measured white blood cell count, differential, creatinine, and liver function tests, which remained normal in all animals (Supplemental Table 2). Total white blood cell count decreased in 6 of 8 animals (mean decrease by 2.1 × 109 ± 2.1 cells/l) (Supplemental Table 2). Neutrophil and lymphocyte counts were also stable (Supplemental Table 2).
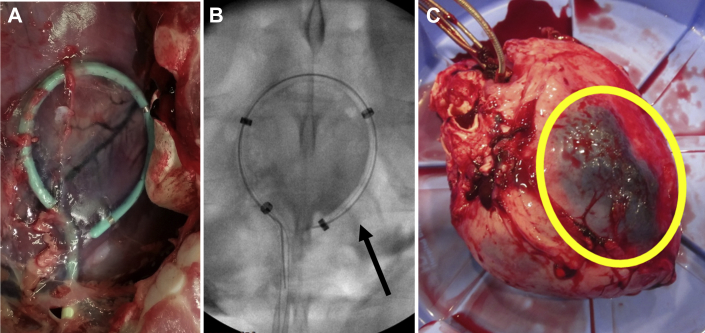
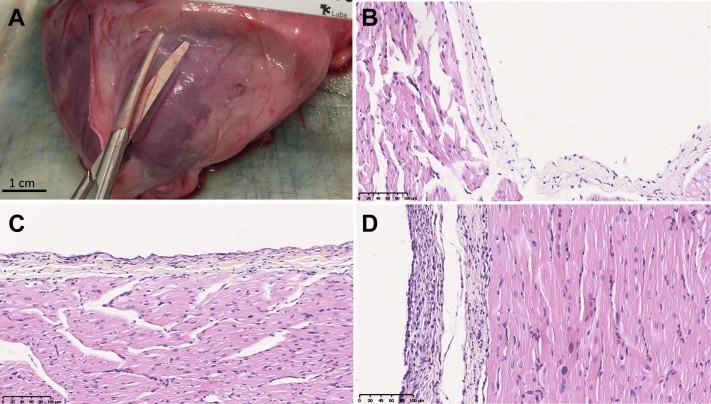
On gross examination, pericardium was thin and translucent 4 to 6 weeks after hydrogel delivery (Figure 4A). Cardiac samples from each chamber were preserved in formalin and stained with hematoxylin and eosin (Figures 4B to 4D). Histologic evaluation showed mostly normal pericardial thickness and cellularity (Figures 4B and 4C). In some animals, in a localized area close to the site of pericardial access near the left ventricular apex, pericardial thickening was observed (Figure 4C). Mononuclear cellular infiltration of the parietal pericardium in this area suggests an inflammatory response or fibroblast proliferation at the sight of pericardial puncture. No abnormalities were seen in the myocardial architecture.
Figure 4.
Histological Appearance of Pericardium and Myocardium 4 Weeks After Pericardial Instrumentation and Gel Delivery Suggest Mild Inflammatory Response
(A) On gross histologic examination, pericardium had normal thin and translucent appearance 4 weeks after hydrogel deliver. After formalin fixation and staining with hematoxylin and eosin (original magnification 10x), pericardium overlying the left atrium (B) and right ventricle (C) showed normal histologic appearance. (D) In some animals a small 1-cm2 area of thickening and increased cellularity was seen at the site of pericardial puncture involving the visceral pericardium.
Discussion
Biocompatible materials are an emerging category of therapeutics to treat CVD. They come in a variety of physical forms (solid, gels) and can be designed to act alone or as carriers for other therapeutics, such as stem cells, miRNAs, and cytokines. The hydrogel used in this study has been shown to improve cardiac function by increasing retention of transplanted mesenchymal stem cells in the heart in small animal studies (17). Although this material does not directly incorporate into the myocardium, other materials nearing or in phase 1 clinical trials work by other mechanisms of action 10, 11, 12, 15, 19. To date, these materials are delivered by techniques or procedures designed for other purposes, such as intracoronary catheters, Noga XP Cardiac Navigation System (Biosense Webster), and open heart surgery. Biomaterial delivery using these techniques may increase the risk of complications, such as arrhythmia, coronary thrombosis, and embolic events, and device failure, such as catheter occlusion. Applying materials to the heart at the time of open heart surgery is feasible but expensive and invasive. There is an unmet need for safe and precise minimally invasive delivery methods for patients not undergoing surgical procedures.
Biomaterial delivery to the pericardial space has several potential advantages over traditional intracoronary, intramyocardial, and intravenous delivery. First, the pericardium is not a vascular space, eliminating the risk of embolization, stroke, and infarction. Second, therapeutics delivered to this space remain focused and concentrated onto the heart by the pericardium, limiting off-target delivery 21, 22. Third, pericardial delivery allows a more liberal gelation time because gel components are not subjected to immediate vascular washout. Fourth, biomaterials may degrade slower in the pericardial space because of lower mechanical forces and reduced access of the immune system. Finally, arrhythmias may be less likely compared with intramyocardial delivery because the electrical conduction and coordination between cardiomyocytes is not disrupted by the material.
With these considerations in mind, we have demonstrated the feasibility and safety of our epicardial hydrogel delivery device using an example of a clinically translatable hydrogel. The delivery device created a temporary compartment within the pericardial space for biomaterial gel delivery that can be positioned using standard cardiac catheterization laboratory fluoroscopy. This allows localization of gel over an anatomic area of interest, such as an infarction. The compartment also allows for complete separation of gel components within the device. The compartment is stable over many minutes securing the timeframe needed for materials to gel. We had no device malfunction because of premature gelation or clogging. Our simple but effective suction mechanism used existing anatomic structures to stabilize the device in vivo while the heart is moving, and to seal the compartment for gelation. Thus this device can be used with other hydrogels not compatible with vascular delivery because of their extended gelation times, or thrombotic or embolic risk.
We have shown a lack of significant hemodynamic and inflammatory effects of pericardial gel delivery. Triggering inflammation in the pericardium is of especially great concern because this area is prone to refractory symptoms and chronic pathologic consequences. Inflammation of the pericardium can cause hemodynamically significant pericardial effusions or thickening and fibrosis of the pericardium leading to cardiac constriction. We undertook careful histologic and hemodynamic studies to assess for these changes. In our study invasive measurements of intracardiac pressures showed stable filling pressures over 4 to 6 weeks. This suggests that pericardial biomaterial delivery will not have negative hemodynamic effects. Histology did show some inflammation localized to the parietal pericardium overlying the pericardial puncture site, whereas the white blood cell count and differential remained stable (Supplemental Table 2). The increased cellularity was localized to a 1-cm2 area that was the site of pericardial puncture and was likely the result of the puncture injury and healing. Other areas of the pericardium and the myocardium appeared normal.
Study limitations
For other pericardial procedures, such as the Lariat device, prophylactic anti-inflammatory medications, such as colchicine, are commonly used. Although reported incidence of pericarditis is low, most patients are prophylactically treated with anti-inflammatory agents, specifically colchicine (28). In one study of the Lariat device, the reported incidence of pericarditis was 5% but 54% of patients were treated with colchicine 23, 28. In the current study, we chose to treat our animals with colchicine based on a preliminary study that showed significant pericardial inflammation when anti-inflammatory agents were not used. Colchicine was selected over systemic steroids because of its efficacy at treating pleuritis and lack of significant side effects compared with steroids or other nonsteroidal anti-inflammatory agents. Future studies are needed to confirm the long-term safety of pericardial biomaterials.
There are other concerns specific to accessing and working within the pericardial space. Our technique is unable to form the delivery compartment in patients without a pericardium (post-surgical or congenitally absent). Large pericardial effusions could potentially be drained before biomaterial delivery, but the safety and device functionality would need to be investigated. The risks of this technique in the acute myocardial infarction period may be elevated because of ischemia-reperfusion-induced inflammation of the pericardium and myocardium. Despite these potential concerns, the safety of pericardial access has been demonstrated by the development of epicardial ablations, the Lariat device, and other procedures. Interventionalists and electrophysiologists have gained experience in this technique 23, 24, 25. Alternatives to percutaneous routs of pericardial access, such as right atrial exit, are under clinical investigation and may be safer in some patients (29). We had no difficulties with percutaneous pericardial access and no instances of myocardial puncture or coronary artery damage.
Many biomaterials close to translation for clinical use have a liquid or gel phase and could be delivered using our device. However there are some biomaterials in development, such as cell sheets and patches, which do not and would not be amenable to our delivery by our method. Our device is also not capable of delivering ultra violet light as required for gelation for some biomaterials, but could be modified to do so. Lastly, most materials have only been tested by intramyocardial or intracoronary injection, through which they are instilled into the myocardium. They may not be as effective if delivered to the epicardium. Although it is unlikely a single technique could accommodate all biomaterials for cardiac application, we believe our technique and device are applicable to many biomaterials. We also wish to advocate for early consideration of the technique for material delivery to facilitate rapid and safe translation of biomaterials into clinical use.
Conclusions
We have developed a novel method for delivering biomaterial gels to the heart through the pericardial space. Our platform can be used with many types of gels and is a new treatment strategy for CVD that may be particularly useful for emerging therapeutics, such as cytokines, miRNAs, and stem cells. Our minimally invasive epicardial hydrogel delivery technique takes advantage of the proximity of the pericardial space to the heart, and its relatively protected status. Minimally invasive, precise, and safe delivery techniques help facilitate translation of biomaterials into clinical use for CVD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Many new technologies, such as stem cells, nucleic acid–based therapies (microRNA, viruses), and biocompatible materials, are under investigation as new treatments for cardiac disease. However, these therapeutics may not be safely delivered to the heart by traditional means. Intracoronary or intramyocardial administration resulted in poor retention of viable stem cells in the heart. Intravascular delivery of biomaterials could cause thrombosis or embolic events.
TRANSLATIONAL OUTLOOK: The technique described here uses the pericardial space as a novel site for therapeutic administration. The technique can be adapted to many types of biomaterials with embedded therapeutics and can serve to focus and localize them over anatomic areas of interest, such as the peri-infarct zone. Early consideration of the mode of administration may improve safety, efficacy, and the speed of translation of new therapeutics into clinical use.
Footnotes
Dr. Campbell and Dr. Levit are listed as inventors on a patent application filed by Emory University on technology related to the delivery device. Dr. Levit is a principal in a startup company, CorAmi LLC, which seeks to commercialize this technology but as of yet has no investment, revenue, or intellectual property. Funded by Coulter Translational Research Partnership, Georgia Research Alliance. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Kamps J.A., Krenning G. Micromanaging cardiac regeneration: targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol. 2016;8:163–179. doi: 10.4330/wjc.v8.i2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai T., Komuro I. Gene and cytokine therapy for heart failure: molecular mechanisms in the improvement of cardiac function. Am J Physiol Heart Circ Physiol. 2012;303:H501–H512. doi: 10.1152/ajpheart.00130.2012. [DOI] [PubMed] [Google Scholar]
- 4.Peng B., Chen Y., Leong K.W. MicroRNA delivery for regenerative medicine. Adv Drug Deliv Rev. 2015;88:108–122. doi: 10.1016/j.addr.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goussetis E., Manginas A., Koutelou M. Intracoronary infusion of CD133+ and CD133−CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279–2283. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 6.Teng C.J., Luo J., Chiu R.C.J., Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Tossios P., Krausgrill B., Schmidt M. Role of balloon occlusion for mononuclear bone marrow cell deposition after intracoronary injection in pigs with reperfused myocardial infarction. Eur Heart J. 2008;29:1911–1921. doi: 10.1093/eurheartj/ehn218. [DOI] [PubMed] [Google Scholar]
- 8.Taimeh Z., Loughran J., Birks E.J., Bolli R. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol. 2013;10:519–530. doi: 10.1038/nrcardio.2013.94. [DOI] [PubMed] [Google Scholar]
- 9.Saharinen P., Eklund L., Pulkki K., Bono P., Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Mann D.L., Lee R.J., Coats A.J. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail. 2015;18:314–325. doi: 10.1002/ejhf.449. [DOI] [PubMed] [Google Scholar]
- 11.Frey N., Linke A., Suselbeck T. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]
- 12.Menasche P., Vanneaux V., Hagege A. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 13.Mann D.L., Kubo S.H., Sabbah H.N. Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn Trial. J Thorac Cardiovasc Surg. 2011;143:1036–1042. doi: 10.1016/j.jtcvs.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Yang H., Bai A. Functional engineered human cardiac patches prepared from nature's platform improve heart function after acute myocardial infarction. Biomaterials. 2016;105:52–65. doi: 10.1016/j.biomaterials.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Seif-Naraghi S.B., Singelyn J.M., Salvatore M.A. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps E.A., Landázuri N., Thulé P.M., Taylor W.R., García A.J. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levit R.D., Landazuri N., Phelps E.A. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelps E.A., Enemchukwu N.O., Fiore V.F. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J.E., Purcell B.P., MacArthur J.W. A Bioengineered hydrogel system enables targeted and sustained intramyocardial delivery of Neuregulin, activating the cardiomyocyte cell cycle and enhancing ventricular function in a murine model of ischemic cardiomyopathy. Circ Heart Fail. 2014;7:619–626. doi: 10.1161/CIRCHEARTFAILURE.113.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tio R.A., Grandjean J.G., Suurmeijer A.J.H., van Gilst W.H., van Veldhuisen D.J., van Boven A.J. Thoracoscopic monitoring for pericardial application of local drug or gene therapy. Int J Cardiol. 2002;82:117–121. doi: 10.1016/s0167-5273(01)00614-3. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Y.F., Sigg D.C., Ujhelyi M.R., Wilhelm J.J., Richardson E.S., Iaizzo P.A. Pericardial delivery of omega-3 fatty acid: a novel approach to reducing myocardial infarct sizes and arrhythmias. Am J Physiol Heart Circ Physiol. 2008;294:H2212–H2218. doi: 10.1152/ajpheart.91502.2007. [DOI] [PubMed] [Google Scholar]
- 22.Moreno R., Waxman S., Rowe K., Verrier R.L. Intrapericardial beta-adrenergic blockade with esmolol exerts a potent antitachycardic effect without depressing contractility. J Cardiovasc Pharmacol. 2000;36:722–727. doi: 10.1097/00005344-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lakkireddy D., Afzal M.R., Lee R.J. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13:1030–1036. doi: 10.1016/j.hrthm.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee S., Herrmann H.C., Wilensky R.L. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE Database. JAMA Intern Med. 2015;175:1104–1109. doi: 10.1001/jamainternmed.2015.1513. [DOI] [PubMed] [Google Scholar]
- 25.Patel M.B., Rasekh A., Shuraih M. Safety and effectiveness of compassionate use of LARIAT(R) device for epicardial ligation of anatomically complex left atrial appendages. J Interv Card Electrophysiol. 2015;42:11–19. doi: 10.1007/s10840-014-9963-2. [DOI] [PubMed] [Google Scholar]
- 26.Phelps E.A., Headen D.M., Taylor W.R., Thule P.M., Garcia A.J. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34:4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorajja P. Invasive hemodynamics of constrictive pericarditis, restrictive cardiomyopathy, and cardiac tamponade. Cardiol Clin. 2011;29:191–199. doi: 10.1016/j.ccl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Gunda S., Reddy M., Nath J. Impact of periprocedural colchicine on postprocedural management in patients undergoing a left atrial appendage ligation using LARIAT. J Cardiovasc Electrophysiol. 2016;27:60–64. doi: 10.1111/jce.12869. [DOI] [PubMed] [Google Scholar]
- 29.Greenbaum A.B., Rogers T., Paone G. Intentional right atrial exit and carbon dioxide insufflation to facilitate subxiphoid needle entry into the empty pericardial space: first human experience. J Am Coll Cardiol EP. 2015;1:434–441. doi: 10.1016/j.jacep.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.