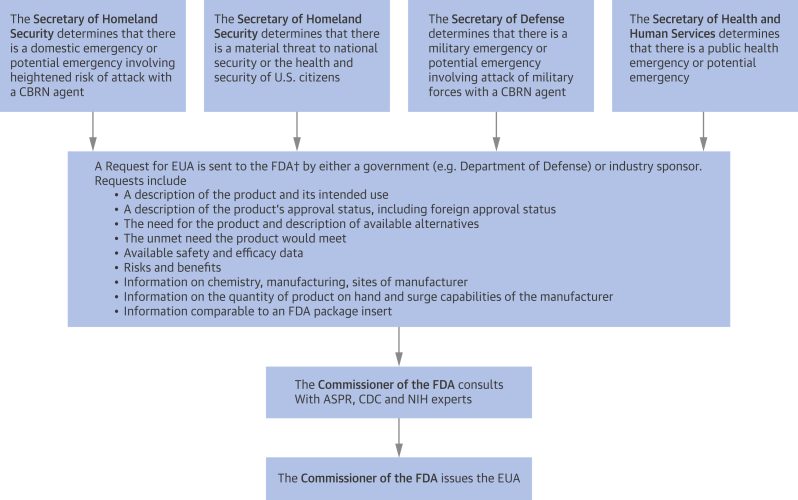
Figure 1.
Process for Issuance of an Emergency Use Authorization
†Submission are to the appropriate Department of the FDA: CDER, CBER, or CDRH. ASPR = HHS Assistant Secretary for Preparedness and Response; CBER = Center for Biologics Evaluation and Research; CBRN = chemical, biological, radiation, or nuclear; CDC = U.S. Centers for Disease Control and Prevention; CDER = Center for Drug Evaluation and Research; CDRH = Center for Devices and Radiological Health; HHS = Health and Human Services; NIH = National Institutes of Health.