ABSTRACT
Two new secondary metabolites, kongiilines A and B (1, 7), and two asperphenamate derivatives, asperphenamates B and C (5–6), together with 16 known compounds (2–4, 8–20), were isolated from Tibetan Plateau fungi Penicillium kongii and Penicillium brasilianum. This is the first report on asperphenamates B and C as naturally occurring compounds, and that aspterric acid is isolated from P. brasilianum for the first time. Their structures were elucidated by different spectroscopic techniques including high-resolution electrospray ionisation mass spectrum, 1D nuclear magnetic resonance (NMR), and 2D NMR as well as electronic circular dichroism. Compounds 4, 5, and 10 exhibited cytotoxicity activities against human colon carcinoma HCT116 cell line with IC50 values of 88.16, 77.68, and 36.92 μM, respectively. Fungi from Tibetan Plateau represent important and rich resources for the investigation of new chemicals.
KEYWORDS: Antitumour cell, moulds, new compounds, structural elucidation, Tibet
Introduction
The genus Penicillium is a major contributor to the production of bioactive molecules. A number of compounds from this genus have been characterised and used for drugs and mycotoxins. For example, the antibiotic penicillin is produced by Penicillium rubens (Houbraken et al. 2011), the immunosuppressive drug mycophenolic acid is produced by Penicillium brevicompactum (Regueira et al. 2011). There were many new compounds that have been found constantly in the following years with impressive anticancer and antifungal activities (Bladt et al. 2013; Kozlovskii et al. 2013; Tang et al. 2015; Koul et al. 2016). And many mycotoxins causing human and animal diseases were produced in some species of Penicillium, such as citreoviridin, citrinin (CIT), ochratoxin A (OTA), patulin (PAT), penitrem A, and penicillic acid (PA) (Lee & Ryu 2015; Oh et al. 2017). Because of the importance of this genus, chemical assessments on more and more Penicillium species have been investigated from different environmental sources. The endophytic fungi (Arunpanichlert et al. 2010; Zhu et al. 2015) and soil fungi (Tansakul et al. 2014; Daengrot et al. 2015) were the most studied fungi in this genus and a number of natural products have been reported to exhibit diverse activities including anti-inflammatory, cytotoxic (Xue et al. 2014), or anti-hepatitis C virus (Kozlovskii et al. 2013; Nishikori et al. 2016). Other strains from extreme conditions also raised the great interest to mine novel structures, such as halotolerant or extremophilic strains (Lu et al. 2008; Stierle et al. 2012),deep sea-derived strains (Li et al. 2013), and marine animal-derived strains (Qi et al. 2013). The resulted compounds cover different biological activities including cytotoxic activity against cancer cell lines (Li et al. 2014; Zhang et al. 2016), potent inhibitory activity against bacteria (Zheng et al. 2016), antifungal activity (Daengrot et al. 2015), inhibiting LPS-induced inflammation (Shin et al. 2016), and inhibitory activity against protease (Sun et al. 2014). In comparison to the above environment-derived Penicillium strains, research on the Penicillium strains from Tibetan Plateau is limited.
Tibetan Plateau, as the highest plateau in the world, is exposed to strong ultraviolet radiation, and has low-temperature and low-oxygen environments. In the past few years, a series of bioactive molecules or new skeletons has been isolated from Tibetan Plateau origin fungi, such as phaeolschidins, with antioxidant activity, from Phaeolus schweinitzii (Abbas et al. 2013; Han et al. 2013), new skeletons, sterhirsutins, with cytotoxic and immunosuppressant activities from Stereum hirsutum (Qi et al. 2014, 2015), anthraquinone derivatives, with antitumour activities, from an Alternaria species (Chen et al. 2014), sarcoviolins, with antioxidative and α‑glucosidase inhibitory activities, from Sarcodon leucopus (Ma et al. 2014a), Gloeophyllins A–J, as cytotoxic ergosteroids, from Gloeophyllum abietinum (Han et al. 2015). Therefore, fungi from Tibetan Plateau represent an important fungal resource for the discovery of novel chemical molecules.
In order to probe the chemical diversity from Tibetan Penicillium, 24 Penicillium strains from Tibetan Plateau were screened and evaluated for secondary metabolite production (data not shown). Based on the high-performance liquid chromatography (HPLC)-UV and/or liquid chromatography–mass spectrometry (LC-MS) analysis, two of them, P. kongii and P. brasilianum with abundant chemical profiles, were selected as targeted strains for further studies. Herein, we described the compound isolation, structural elucidation, and bioactivity evaluations for new compounds.
Materials and methods
Fungal materials
Plant leaf samples were collected from growing trees and kept in sterilised plastic bags. Isolation of phylloplane fungi was according to Nakase and Takashima (1993). Penicillium strain XZ135R was identified as P. kongii (isolated from leaves of Cotoneaster sp., Gongbujiangda, Linzhi, Tibet, China), and XZ94 was identified as P. brasilianum (isolated from leaves of an unidentified plant, Cuona, Shannan, Tibet, China) according to beta-tubulin gene (BenA, GenBank No. MF036174) and calmodulin gene (CaM, GenBank No. MF039288). The method of DNA extraction, the primers for amplifying the aforesaid two genes (BenA and CaM), and the polymerase chain reactions followed the methods of Wang and Wang (2013). The phylogenetic trees were drawn using maximum likelihood method and subjected to 1000 bootstrap replications (Frisvad & Samson, 2004) and supplied as Supporting Information Figures S1 and S2. The strains were stocked on slants of potato dextrose agar (PDA) at 4°C and deposited in China General Microbiological Culture Collection of Institute of Microbiology, Chinese Academy of Sciences, Beijing, China as AS3.15332 and AS3.15722, respectively, and they were also maintained at the authors’ laboratory and will be supplied upon request for educational or scientific purpose. The two strains were activated and cultured on plates of PDA at 25°C for 7 d. Then, cultures were transferred to the modified YES solid medium (2% yeast extracts (BD BactoTM), 15% sucrose, and 1.5% agar) with 20.0 mL on each plate at 25°C for 7 d (Frisvad 1981). For the large-scale fermentations, 12 L YES solid cultures of P. kongii and 3 L YES solid cultures of P. brasilianum were performed, respectively. They were growing at 25°C for 7 d before harvest.
Analysis methods
Analytical HPLC was conducted with a Waters HPLC system (Waters e2695, Waters 2998, Photodiode Array Detector) using an ODS column (C18, 250 mm × 4.6 mm, YMC Pak, 5 μM) with a flow rate of 1 mL/min. Fresh extracts were dissolved in methanol before separated on a linear gradient of MeOH:H2O (0.1% formic acid) at a flow rate of 1 mL/min. Fresh extracts from screened strains were detected for 30 min using a linear gradient of 20–100% (0–20 min), 100% MeOH (20–25 min), 20% MeOH (25–30 min). LC-MS analysis method was used as keeping consistent with analytical HPLC.
General experimental procedures
The optical rotations were measured on a Perkin-Elmer 241 polarimeter (Waltham, USA) and UV spectra were determined on a Thermo Genesys-10S UV–Vis spectrophotometer (Madison, USA). Electronic circular dichroism (ECD) spectra were recorded on a JASCO J-815 spectropolarimeter (Tokyo, Japan). NMR spectra were recorded on a Bruker Avance-500 spectrometer using tetramethyl silicane (TMS) as internal standard, and chemical shifts were recorded as δ values. High-resolution electrospray ionisation mass spectrum (HR-ESI-MS) and LC-MS were utilised on an Agilent Accurate-Mass-QTOF LC-MS 6520 instrument. Sephadex LH-20 was purchased from GE Healthcare. TLC was carried out on Silica gel HSGF254 and the spots were visualised by spraying with 10% H2SO4 and heating. Silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and ODS (Lobar, 40–63 mm, Merck, Darmstadt, Germany) were used for column chromatography (CC). RP-HPLC separations were conducted using a Shimadzu LC-6AD liquid chromatograph with a YMC-PACK ODS-A column (250 mm × 10 mm, 5 µm) and a Shimadzu SPD-20A VP UV–Vis detector with a flow rate of 3 mL/min. Solvents used for extraction and chromatographic separation were of analytical grades.
Extraction and isolation
The fermented YES cultures were extracted thoroughly with ethyl acetate (2 × 12 L and 2 × 3 L, respectively) under ultrasonic with 100 Hz for 1 h, and then, organic phases were evaporated to dryness under reduced pressure to afford two crude residues (9.8 g and 3.0 g, respectively). The 9.8-g residues of P. kongii were subjected to a silica gel CC using a gradient elution with CH2Cl2–MeOH (100:0–0:100, v/v) to give six fractions (fractions A–F). Fr. A (1.87 g) was subjected to Sephadex LH-20 CC eluting with MeOH to afford two subfractions (Fr. A-1–Fr. A-2). Fr. A-1 was repeatedly recrystallised in CHCl3–MeOH (1:1) to give compound 4 (180.0 mg) and 10 (220.0 mg). Compound 3 (30 mg, tR 23.5 min) was purified from Fr. A-2 by semi-preparative HPLC (MeOH–H2O, 75:25). Fr. B (0.89 g) was divided into three subfractions Fr. B-1–Fr. B-3 by Sephadex LH-20 column (MeOH). Fr. B-1 was further purified by semi-preparative HPLC (MeOH–H2O, 70:30) to afford 8 (5.9 mg, tR 11.9 min), 2 (5.6 mg, tR 14.3 min), 1 (4.3 mg, tR 19.8 min), 5 (10.0 mg, tR 23.1 min), and 6 (1.8 mg, tR 29.8 min). Compounds 9 (7.4 mg, tR 18.5 min) and 11 (4.7 mg, tR 22.4 min) were isolated from Fr. B-3 by semi-preparative HPLC eluting with MeOH–H2O (45:55). Fr. C (1.03 g) was passed through a Sephadex LH-20 column (MeOH) and further purified by semi-preparative HPLC (MeOH–H2O, 60:40) to afford 7 (1.7 mg, tR 15.9 min).
The 3.0-g extracts of P. brasilianum were subjected to CC on reversed-phase ODS using a gradient system of MeOH–H2O (from 40% to 100%) to afford seven fractions (Fr. G–Fr. M). Fr. H (0.5 g) was divided into three subfractions Fr. H-1–Fr. H-3 by Sephadex LH-20 column eluting with MeOH. Compounds 18 (12.4 mg, tR 14.6 min), 12 (2.7 mg, tR 17.5 min), and 15 (4.9 mg, tR 22.9 min) were purified from Fr. H-1 by semi-preparative HPLC (MeOH–H2O, 60:40). Fr. J (0.4 g) was passed through a Sephadex LH-20 column (MeOH) and further purified by semi-preparative HPLC (MeOH–H2O, 65:35) to afford 16 (0.9 mg, tR 21.4 min) and 17 (3.0 mg, tR 24.5 min). Fr. K (0.3 g) was chromatographed on a Sephadex LH-20 column eluting with MeOH to give 19 (2.3 mg) and 20 (13.7 mg). Fr. M (0.8 g) was subjected to a silica gel column eluting with petroleum ether–acetone, followed by recrystallisation in CHCl3 to give 13 (280 mg) and 14 (60 mg). Kongiiline A (1), white needles.[a]25D–65 (c 0.1, MeOH). UV (MeOH) λmax (log ε) 238 (2.80) nm; 273 (0.72) nm.1H NMR (500 MHz, methanol-d4) and 13C NMR (125 MHz, methanol-d4) see Table 1. HR-ESI-MS m/z 373.2013 [M + H]+ (calcd for C22H29O5, 373.2010).
Table 1.
1H NMR and 13C NMR data for compounds 1 and 7.
| 1a |
7b |
||||
|---|---|---|---|---|---|
| Position | δC | δH (J Hz) | Position | δC | δH (J Hz) |
| 1a | 43.8 | 1.12, m | 2 | 161.6 | |
| 1b | 1.18, m | 3 | 87.5 | 5.65, s | |
| 2a | 18.7 | 1.53, m | 4 | 169.1 | |
| 2b | 1.76, m | 5 | 149.2 | ||
| 3a | 38.3 | 1.20, m | 6 | 107.7 | 7.26, s |
| 3b | 1.69, m | 7 | 131.4 | ||
| 4 | 39.2 | 8 | 143.7 | ||
| 5 | 45.9 | 1.80, m | 9 | 132.1 | |
| 6 | 69.3 | 5.67, m | 10 | 105.9 | |
| 7a | 28.8 | 2.12, m | 11 | 65.4 | 4.67, d (6.5) |
| 7b | 2.47, m | 12 | 119.8 | 5.47, d (6.5) | |
| 8 | 37.0 | 2.87, t (8.0) | 13 | 137.3 | |
| 9 | 50.7 | 2.40, m | 14 | 18.1 | 1.74, s |
| 10 | 35.7 | 15 | 25.5 | 1.76, s | |
| 11a | 69.4 | 4.28,dd (5.5,10) | 16 | 61.2 | 4.80, d (4.0) |
| 11b | 4.47, d (9.5) | 17 | 56.7 | 3.94, s | |
| 12 | 181.2 | ||||
| 13 | 20.3 | 0.88, s | |||
| 14a | 71.2 | 3.03, d (6.5) | |||
| 14b | 3.56, d (6.0) | ||||
| 15 | 19.1 | 1.43, s | |||
| 1′ | 168.1 | ||||
| 2′ | 132.0 | ||||
| 3′ | 130.6 | 7.95,dd (1.5,7.0) | |||
| 4′ | 129.6 | 7.48, d (8.0) | |||
| 5′ | 134.1 | 7.60, t (7.5) | |||
| 6′ | 129.6 | 7.47, d (7.5) | |||
| 7′ | 130.6 | 7.97, dd (1.5,7.0) | |||
aRecorded in MeOD. brecorded in DMSO-d6.
Asperphenamate B (5), white powder.[a]25D–124 (c 0.1, MeOH). UV (MeOH) λmax (log ε) 237 (2.38) nm; 272 (0.26) nm. ECD (MeOH) λmax (Δε) 234 (−9.28) nm. 1H NMR (500 MHz, DMSO-d6) and 13C NMR (125 MHz, DMSO-d6) see Table 2. HR-ESI-MS m/z 523.2232 [M + H]+ (calcd for C32H31N2O5, 523.2227).
Table 2.
1H NMR and 13C NMR data for compounds 5 and 6.
| 5a |
6b |
|||
|---|---|---|---|---|
| Position | δC | δH (J Hz) | δC | δH (J Hz) |
| 1 | 172.2 | 173.3 | ||
| 2 | 54.8 | 4.87, dd (6.5,11.5) | 56.3 | 4.76, dd (6.0,9.0) |
| 3a | 36.9 | 3.12, dd (6.5,14.0) | 37.9 | 3.13, dd (9.0,14.0) |
| 3b | 3.19, dd (6.5,14.0) | 3.27, dd (6.0,9.0) | ||
| 4 | 127.4 | 138.6 | ||
| 5 | 130.5 | 7.04, d (8.5) | 130.5 | 7.26, m |
| 6 | 116.0 | 6.76, d (8.5) | 129.5 | 7.24, m |
| 7 | 155.5 | 127.9 | 7.22, m | |
| 8 | 116.0 | 6.76, d (8.5) | 129.5 | 7.24, m |
| 9 | 130.5 | 7.04, d (8.5) | 130.5 | 7.26, m |
| 10 | 167.7 | 170.3 | ||
| 11 | 134.2 | 125.7 | ||
| 12 | 127.3 | 7.68, d (7.5) | 130.2 | 7.60, d (8.5) |
| 13 | 128.8 | 7.30, t (7.5) | 116.1 | 6.76, d (8.5) |
| 14 | 131.6 | 7.42, t (7.5) | 162.3 | |
| 15 | 128.8 | 7.30, t (7.5) | 116.1 | 6.76, d (8.5) |
| 16 | 127.3 | 7.68, d (7.5) | 130.2 | 7.60, d (8.5) |
| 1′a | 65.5 | 4.03, dd (3.5,11.5) | 66.7 | 4.11, dd (6.0,11.0) |
| 1′b | 4.50, dd (4.5,11.0) | 4.41, dd (4.5,11.0) | ||
| 2′ | 50.5 | 4.60, m | 51.9 | 4.56, m |
| 3′a | 37.4 | 2.90, dd (7.0,14.0) | 37.4 | 2.88, dd (8.5,14.0) |
| 3′b | 3.00, dd (6.5,14.0) | 2.93, dd (6.0,14.0) | ||
| 4′ | 137.2 | 139.1 | ||
| 5′ | 129.4 | 7.22, d (8.0) | 130.3 | 7.25, m |
| 6′ | 128.9 | 7.30, t (7.5) | 129.4 | 7.23, m |
| 7′ | 127.0 | 7.30, t (7.5) | 127.6 | 7.20, m |
| 8′ | 128.9 | 7.24, t (7.5) | 129.4 | 7.23, m |
| 9′ | 129.4 | 7.22, d (8.0) | 130.3 | 7.25, m |
| 10′ | 167.6 | 170.1 | ||
| 11′ | 133.4 | 135.7 | ||
| 12′ | 127.2 | 7.65, d (7.5) | 128.4 | 7.67, d (7.5) |
| 13′ | 128.6 | 7.38, t (7.5) | 127.2 | 7.37, d (7.5) |
| 14′ | 132.2 | 7.50, t (7.5) | 132.6 | 7.49, t (7.5) |
| 15′ | 128.6 | 7.38, t (7.5) | 127.2 | 7.37, d (7.5) |
| 16′ | 127.2 | 7.65, d (7.5) | 128.4 | 7.67, d (7.5) |
aRecorded in DMSO-d6.brecorded in MeOD.
Asperphenamate C (6), white powder.[a]25D–89 (c 0.1, MeOH). UV (MeOH) λmax (log ε) 242 (1.20) nm. ECD (MeOH) λmax (Δε) 234 (−3.89) nm. 1H NMR (500 MHz, methanol-d4) and 13C NMR (125 MHz, methanol-d4) see Table 2. HR-ESI-MS m/z 523.2231 [M + H]+ (calcd for C32H31N2O5, 523.2227).
Kongiiline B (7), white needles. UV (MeOH) λmax (log ε) 248 (0.32) nm; 306 (0.54) nm. 1H NMR (500 MHz, DMSO-d6) and 13C NMR (125 MHz, DMSO-d6) see Table 1. HR-ESI-MS m/z 307.1172 [M + H]+ (calcd for C16H19O6, 307.1176).
Cytotoxicity assays
Cytotoxic activity against HCT116 human colon carcinoma cells was assayed according to the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay method (Ma et al. 2014b). Cells were incubated with tested compounds (DMSO as solvent) at 37°C in a humidified atmosphere of 5% CO2 95% air for 72 h. Each well was added 50 μL of MTT/medium solution (0.5 mg/mL), and cells were incubated for another 4 h. After removing the MTT/medium, 100 μL of DMSO was added to each well. The plate was shaken to dissolve the precipitates, and activity was measured at 540 nm using a microplate reader. The inhibition rates were calculated and plotted versus test concentrations to afford the IC50 (± SD) for three independent experiments, each was carried out in triplicate. Taxol was used as the reference substance that showed cytotoxicity against HCT116 human colon carcinoma with IC50 value of 0.98 ± 0.12 μM.
Results and discussion
To characterise the compounds from P. kongii and P. brasilianum, the fermentations on YES media were carried out. The organic extracts were fractionated by ODS and followed from Sephadex LH-20 CC (see Methods). The subfractions containing the targeted metabolites were then selected for further purification. After the semi-preparative reversed-phase HPLC separation step, we isolated 20 compounds (1–20) including 2 new compounds which were named kongiilines A and B (1, 7) and 2 new natural compounds which were named asperphenamates B and C (5, 6) (Figures 1 and 2). The two compounds of asperphenamates B and C (5, 6), as asperphenamate derivatives, were synthesised in 2012 (Yuan et al. 2012), but they were isolated from fungi as the natural compounds for the first time.
Figure 1.
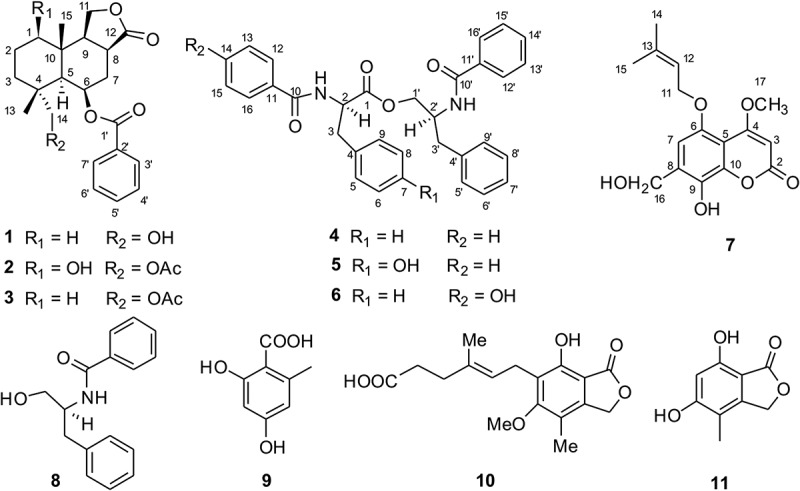
The chemical structures of compounds 1–11 from P. kongii.
Figure 2.
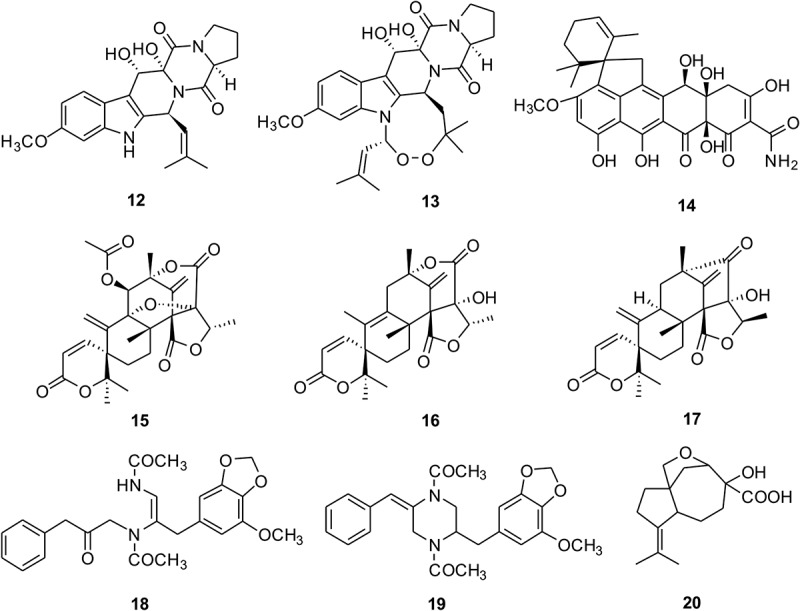
The chemical structures of compounds 12–20 from P. brasilianum.
Kongiiline A (1) was isolated as white needles. The molecular formula C22H29O5 of 1 was determined by HR-ESI-MS at m/z 373.2013 [M + H]+ (calcd 373.2010). 1H NMR spectrum (Table 1) indicated the presence of two tertiary methyls (δ 0.88, 1.43), two oxymethylenes (δ 3.03, d, J = 6.5 Hz; 3.56, d, J = 6.0 Hz; 4.28, dd, J = 10.0, 5.5 Hz; 4.47, d, J = 9.5 Hz), one oxymethine (δ 5.67, m), and five aromatic protons, respectively. 13C NMR and heteronuclear singular quantum correlation (HSQC) spectra showed a total of 22 carbon signals that could be classified into 2 carbonyl, 6 olefinic carbons, 3 sp3 quaternary carbon, 5 sp3 methines (including 2 oxymethines), 4 sp3 methylenes, and 2 methyls (Table 1). Analysing the 1H and 13C NMR spectra indicated that 1 possessed the drimane-type sesquiterpenoid skeleton, which showed signals similar to those for 1-deoxypebrolide (McCorkindale et al. 1978). The sole difference was that one acetyl group is replaced by a hydroxyl group at C-14 in 1. This was corroborated by analysis of the heteronuclear multiple bond correlation (HMBC) spectrum, in which long-range correlations of H-5 and H3-13 with C-14 (δ 71.2), and of H-14 to C-4 (δ 39.2), C-5 (δ 45.3) and C-13 (δ 20.3), indicated that the hydroxyl group located at c-14(δ -71.2) (Figure 3). The relative configuration of 1 was further confirmed by analysis of the coupling constants and nuclear overhauser effect spectroscopy (NOESY) spectrum, in which the correlations of H-14/H-5 and H-6, H-5/H-9, H-6/H-8, and H3-13/H3-15 indicated an α-orientation of H-5, H-6, H-8, H-9, H2-14 and a β-orientation of H3-13 and H3-15, respectively. The structure of 1 was finally named as1-deoxy-14-desacetylpebrolide.
Figure 3.
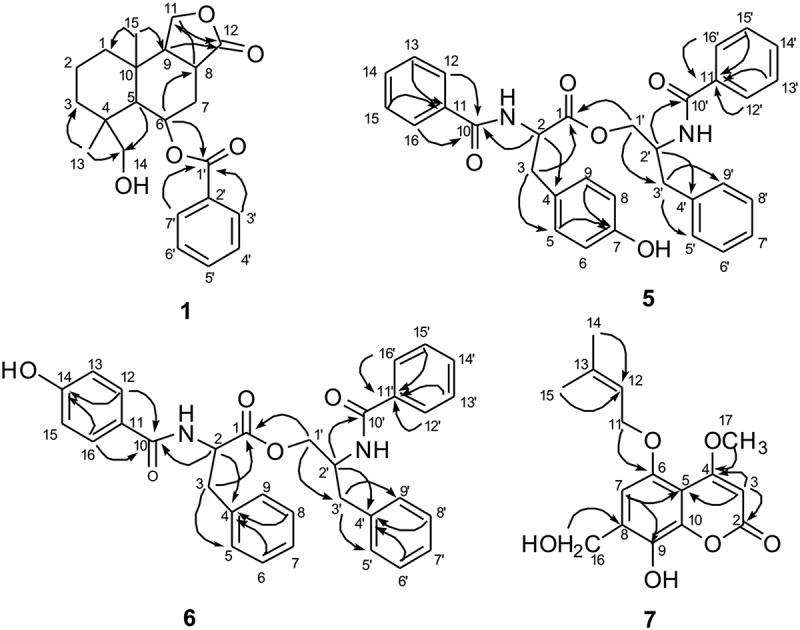
The key HMBC correlations of compounds 1, 5–7.
Asperphenamate B (5) was isolated as white powder, and the molecular formula was determined to be C32H30N2O5 based on the HR-ESI-MS at m/z 523.2232 [M + H]+ (calcd for C32H31N2O5, 523.2227). The NMR data (Table 2) suggested that the structure of compound 5 was likely an amino-acid ester, which was similar to that of asperphenamate (Fukuda et al. 2011). The difference between the two compounds was that an additional hydroxyl group was located at 5. From the HMBC spectrum analysis, the correlations from H-2 to C-1 (δ 172.2), C-3 (δ 36.9), and C-10 (δ 167.7) and from H-3 to C-1 (δ 172.2), C-5 (δ 130.5), and C-9 (δ 130.5) and from H-5/H-9 to C-3 (δ 36.9), C-4 (δ 127.4), and C-7 (δ 155.5) and from H-12/H-16 to C-10 (δ 167.7) indicated the partial structure with a N-benzoyl-tyrosine group (Figure 3). The partial structure of N-benzoyl-phenylalaninol was defined by the HMBC correlations from H-2′ to C-1′ (δ 65.5) and C-10′ (δ 167.6), and from H-3′ to C-1′ (δ 65.5), C-5′ (δ 129.4), and C-9′ (δ 129.4) and from H-1′ to C-2′ (δ 50.5) and C-3′ (δ 37.4), and from H-12′/H-16′ to C-10′ (δ 167.6) (Figure 3). In addition, its absolute configuration was established from the negative Cotton effect at 234 nm in the ECD spectrum, which was consistent with a known compound asperphenamate (McCorkindale et al. 1978). Furthermore, the specific rotation of 5 was similar to that of asperphenamate. Therefore, the three-dimensional (3D) configurations of 2 and 2′ position are same between compound 5 and asperphenamate, and the structure of 5 was defined as N-benzoyl-tyrosine-2-benzoylamino-3-phenyl propyl ester.
Asperphenamate C (6) has the same molecular formula C20H30N2O5 with m/z 523.2231 [M + H]+ (calcd for C32H31N2O5, 523.2227) as 5 based on the HR-ESI-MS analysis. Comparison of the 1D and 2D NMR data between 6 and 5 indicated that the gross structure of compound 6 is the same as that of 5 with the difference of the location of one of the hydroxyl groups (Table 2). The hydroxyl group was determined at C-14 from the observation of the HMBC correlations from H-12/H-16 to C-10 (δ 170.3), C-13/C-15 (δ 116.1), and C-14 (δ 162.3). The absolute configuration of 6 was determined by the ECD spectrum, which showed the similar Cotton effect with asperphenamate. Furthermore, the specific rotation of 5 was similar to that of asperphenamate, which was consistent with asperphenamate (McCorkindale et al. 1978). Thus, the 3D configurations of 2 and 2′ position are same between compound 6 and asperphenamate, and compound 6 was defined as N-p-phydroxybenzoyl-phenylalanine-2-benzoylamino-3-phenyl propyl ester. To the best of our knowledge, compound 6 was isolated from nature for the first time.
Kongiiline B (7) was obtained as white needles. Its molecular formula was determined to be C16H18O6 with m/z 307.1172 [M + H]+ (calcd for C16H19O6, 307.1176) based on HR-ESI-MS data. Analyses of the 1H, 13C NMR data (Table 1) and HSQC spectra of 7 indicated that it belongs to isopentenylcoumarin (Fukuda et al. 2011), which was bearing substituent groups of methylol, methoxy, and hydroxyl. The prenyloxy group was attached to C-5 by the analysis of HMBC correlations (Figure 3) from H2-11 to C-5 (δ 149.2). The relative configuration of 7 was further confirmed by analysis of the coupling constants and NOESY spectrum. The long-range correlations from H2-16 to C-6 (δ 107.7), C-7 (δ 131.4) and C-8 (δ 143.7), and from H3-17 to C-4 (δ 169.1) indicated that methylol group was at C-7, hydroxyl group at C-9, and methoxy group at C-4, respectively. Thus, the structure of compound 7 was established as 3-methoxy-7-methylol-8-hydroxy-5- isopentenylcoumarin.
The other known compounds isolated from P. kongii including pebrolide (Mccorkindale et al. 1981) (2), 1-deoxypebrolide (Mccorkindale et al. 1981) (3), asperphenamate (McCorkindale et al. 1978) (4), N-benzoyl-phenylalaninol (McCorkindale et al. 1978) (8), orsellinic acid (Fang et al. 2012) (9), mycophenolic acid (Makara et al. 1996) (10), and 5, 7-dihydroxy-4-methylphthalide (Fujimoto et al. 1999) (11), and the other known compounds isolated from P. brasilianum including 12, 13-dihydroxyfumitremorgin C (Inokoshi et al. 2013) (12), verruculogen (Afiyatullov et al. 2004) (13), viridicatumtoxin (Inokoshi et al. 2013) (14), dehydroaustin (Hayashi et al. 1994) (15), austinolide (Lo et al. 2012) (16), neoaustin (Hayashi et al. 1994) (17), brasiliamide A (Fujita et al. 2002) (18), brasiliamide C (Fujita & Hayashi 2004) (19), and aspterric acid (Shimada et al. 2002) (20), were determined by comparison of those reported spectroscopic data. Among these, aspterric acid is reported from P. brasilianum for the first time. There was obvious difference on metabolic profiles between the P. brasilianum strain from plant leaves of Tibetan Plateau environments and those as endophytes (Tang et al. 2015) and soil isolates (Fujita & Hayashi, 2004).
The isolated compounds were tested for their cytotoxicities against human cancer cells (Table 3). Hydroxylated asperphenamates asperphenamate B (5) and asperphenamate C (6) displayed very weak cytotoxicity activities against human cancer cell line HCT116. The IC50 values for 5 and 6 were detected at 88.16 and 77.68 μM, respectively (Table 3). Known compound mycophenolic acid (10) exhibited activities against HCT116 with IC50 value of 36.92 μM (Table 3). This compound has shown previously outstanding anti-proliferative immunosuppressive activity and was used as drug for organ transplant patients. The other tested compounds were inactive.
Table 3.
Cytotoxic activities against HCT116 of 1–11.
| Compound | IC50 ± SD (μM) | Compound | IC50 ± SD (μM) |
|---|---|---|---|
| 1 | >100 | 7 | >100 |
| 2 | >100 | 8 | >100 |
| 3 | >100 | 9 | >100 |
| 4 | 88.16 ± 5.52 | 10 | 36.92 ± 1.96 |
| 5 | 77.68 ± 2.84 | 11 | >100 |
| 6 | 91.72 ± 8.31 | Taxol | 0.98 ± 0.12 |
Among these compounds, asperphenamate is composed of two amino acids via ester bond connection and has been reported with anticancer activity (Pomini et al. 2006). Subsequently, several derivatives were synthesised for improving the solubility and activity. For example, asperphenamate derivatives 1a and 1c, as a pair of isomers, showed activities against T47D, MDA-MB231, and HL60 cell lines, and 1c exhibited the most potent activities against breast cancer cell lines T47D and MDA-MB231 (IC50 = 8.2 and 11.9 μM, respectively) (Yuan et al. 2010). Asperphenamate derivative IM23b, benzyl modified at C15 of asperphenamate, showed the greatest potency in human breast cancer MCF-7 cells (Yuan et al. 2012). This compound was also found in P. brevicompactum. The phylogenetic and morphological study showed that P. kongii belongs to Penicillium section brevicompacta and has some kinship with P. brevicompactum (Wang & Wang 2013). Recently, genome of P. brasilianum has been sequenced (Horn et al. 2015), which provides the opportunity to elucidate its biosynthetic pathway.
Funding Statement
This work was supported by the National Natural Science Foundation of China under: [Grant Number 81502964] to Z.G.L. and [Grant Number 31270539] to L.W., the State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College under Grant GTZK201506. W.B.Y. is a scholar of the “100 Talents Project” of CAS.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under: [Grant Number 81502964] to Z.G.L. and [Grant Number 31270539] to L.W., the State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College under Grant GTZK201506. W.B.Y. is a scholar of the “100 Talents Project” of CAS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/21501203.2017.1331937.
References
- Abbas A, Coghlan A, O’Callaghan J, Garcia-Estrada C, Martin JF, Dobson ADW.. 2013. Functional characterization of the polyketide synthase gene required for ochratoxin A biosynthesis in Penicillium verrucosum. Int J Food Microbiol. 162:311. [DOI] [PubMed] [Google Scholar]
- Afiyatullov SS, Kalinovskii AI, Pivkin MV.. 2004. Fumitremorgins from the marine isolate of the fungus Aspergillus fumigatus. Chem Nat Compd. 40:615–617. [Google Scholar]
- Arunpanichlert J, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Tewtrakul S, Rungjindamai N, Sakayaroj J. 2010. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Chem Pharm Bull (Tokyo). 58:1033–1036. [DOI] [PubMed] [Google Scholar]
- Bladt TT, Frisvad JC, Knudsen PB, Larsen TO. 2013. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules. 18:11338–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Shen Q, Zhu X, Lin Y. 2014. The anthraquinone derivatives from the fungus Alternaria sp. XZSBG-1 from the saline lake in Bange, Tibet, China. Molecules. 19:16529–16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daengrot C, Rukachaisirikul V, Tansakul C, Thongpanchang T, Phongpaichit S, Bowornwiriyapan K, Sakayaroj J. 2015. Eremophilane sesquiterpenes and diphenyl thioethers from the soil fungus Penicillium copticola PSU-RSPG138. J Nat Prod. 78:615–622. [DOI] [PubMed] [Google Scholar]
- Fang MJ, Fang H, Li WJ, Huang DM, Wu Z, Zhao YF. 2012. A new diphenyl ether from Phoma sp. strain, SHZK-2. Nat Prod Res. 26:1224–1228. [DOI] [PubMed] [Google Scholar]
- Frisvad JC. 1981. Physiological criteria and mycotoxin production as AIDS in identification of common asymmetric penicillia. Appl Environ Microbiol. 41:568–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Samson RA. 2004. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud Mycol. 49:1–173. [Google Scholar]
- Fujimoto H, Fujimaki T, Okuyama E, Yamazaki M. 1999. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem Pharm Bull (Tokyo). 47:1426–1432. [DOI] [PubMed] [Google Scholar]
- Fujita T, Hayashi H. 2004. New brasiliamide congeners, brasiliamides C, D and E, from Penicillium brasilianum Batista JV-379. Biosci Biotechnol Biochem. 68:820–826. [DOI] [PubMed] [Google Scholar]
- Fujita T, Makishima D, Akiyama K, Hayashi H. 2002. New convulsive compounds, brasiliamides A and B, from Penicillium brasilianum Batista JV-379. Biosci Biotech Biochem. 66:1697–1705. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Sudoh Y, Tsuchiya Y, Okuda T, Fujimori F, Igarashi Y. 2011. Marianins A and B, prenylated phenylpropanoids from Mariannaea camptospora. J Nat Prod. 74:1327–1330. [DOI] [PubMed] [Google Scholar]
- Han JJ, Bao L, He LW, Zhang XQ, Yang XL, Li SJ, Yao YJ, Liu HW. 2013. Phaeolschidins A-E, five hispidin derivatives with antioxidant activity from the fruiting body of Phaeolus schweinitzii collected in the Tibetan Plateau. J Nat Prod. 76:1448–1453. [DOI] [PubMed] [Google Scholar]
- Han JJ, Bao L, Tao QQ, Yao YJ, Liu XZ, Yin WB, Liu HW. 2015. Gloeophyllins A-J, cytotoxic ergosteroids with various skeletons from a Chinese Tibet fungus Gloeophyllum abietinum. Org Lett. 17:2538–2541. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Mukaihara M, Clardy J. 1994. Acetoxydehydroaustin, a new bioactive compound, and related compound neoaustin from Penicillium sp. MG–11. Biosci Biotechnol Biochem. 58:334–338. [Google Scholar]
- Horn F, Linde J, Mattern DJ, Walther G, Guthke R, Brakhage AA, Valiante V. 2015. Draft genome sequence of the fungus Penicillium brasilianum MG11. Genome Announc. 3:pii: e00724–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. 2011. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus. 2:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokoshi J, Nakamura Y, Hongbin Z, Uchida R, Nonaka K, Masuma R, Tomoda H. 2013. Spirohexalines, new inhibitors of bacterial undecaprenyl pyrophosphate synthase, produced by Penicillium brasilianum FKI-3368. J Antibiot (Tokyo). 66:37–41. [DOI] [PubMed] [Google Scholar]
- Koul M, Meena S, Kumar A, Sharma PR, Singamaneni V, Riyaz-Ul-Hassan S, Hamid A, Chaubey A, Prabhakar A, Gupta P, et al. 2016. Secondary metabolites from endophytic fungus Penicillium pinophilum induce ros-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Med. 82:344–355. [DOI] [PubMed] [Google Scholar]
- Kozlovskii AG, Zhelifonova VP, Antipova TV. 2013. Fungi of the genus Penicillium as producers of physiologically active compounds (review). Appl Biochem Microbiol. 49:5–16. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ryu D. 2015. Advances in mycotoxin research: public health perspectives. J Food Sci. 80:T2970–T2983. [DOI] [PubMed] [Google Scholar]
- Li CS, Li XM, Gao SS, Lu YH, Wang BG. 2013. Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44. Mar Drugs. 11:3068–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang X, Lin YY, Yuan J, Lu YJ, Zhu X, Li J, Li MF, Lin YC, He JG, et al. 2014. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303#. Fitoterapia. 97:241–246. [DOI] [PubMed] [Google Scholar]
- Lo HC, Entwistle R, Guo CJ, Ahuja M, Szewczyk E, Hung JH, Chiang YM, Oakley BR, Wang CC. 2012. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J Am Chem Soc. 134:4709–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZY, Lin ZJ, Wang WL, Du L, Zhu TJ, Fang YC, Gu QQ, Zhu WM. 2008. Citrinin dimers from the halotolerant fungus Penicillium citrinum B-57. J Nat Prod. 71:543–546. [DOI] [PubMed] [Google Scholar]
- Ma K, Han J, Bao L, Wei T, Liu HW. 2014a. Two sarcoviolins with antioxidative and alpha-glucosidase inhibitory activity from the edible mushroom Sarcodon leucopus collected in Tibet. J Nat Prod. 77:942–947. [DOI] [PubMed] [Google Scholar]
- Ma K, Ren J, Han J, Bao L, Li L, Yao Y, Sun C, Zhou B, Liu HW. 2014b. Ganoboninketals A-C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense. Pat. J Nat Prod. 77:1847–1852. [DOI] [PubMed] [Google Scholar]
- Makara GM, Keserû GM, Kajtár-Peredy M, Anderson WK. 1996. Nuclear magnetic resonance and molecular modeling study on mycophenolic acid: implications for binding to inosine monophosphate dehydrogenase. J Med Chem. 39:1236–1242. [DOI] [PubMed] [Google Scholar]
- McCorkindale NJ, Baxter RL, Roy TP, Shields HS, Stewart RM, Hutchinson SA. 1978. Synthesis and chemistry of N-benzoyl-O- [N’-benzoyl-l-phenylalanyl]-l-phenylalaninol, the major mycelial metabolite of Penicillium canadense. Tetrahedron. 34:649–653. [Google Scholar]
- Mccorkindale NJ, Calzadilla CH, Hutchinson SA, Kitson DH, Ferguson G, Campbell IM. 1981. The structure and chemistry of pebrolide, desacetylpebrolide and 1-deoxypebrolide, sesquiterpene benzoates from Penicillium brevicompactum. Tetrahedron. 37:649–653. [Google Scholar]
- Nakase T, Takashima M. 1993. A simple procedure for the high frequency isolation of new taxa of ballistosporous yeasts living on the surfaces of plants. RIKEN Review. 3:33–34. [Google Scholar]
- Nishikori S, Takemoto K, Kamisuki S, Nakajima S, Kuramochi K, Tsukuda S, Iwamoto M, Katayama Y, Suzuki T, Kobayashi S, et al. 2016. Anti-hepatitis C virus natural product from a fungus, Penicillium herquei. J Nat Prod. 79:442–446. [DOI] [PubMed] [Google Scholar]
- Oh SY, Cedergreen N, Yiannikouris A, Swamy HV, Karrow NA. 2017. Assessing interactions of binary mixtures of Penicillium mycotoxins (PMs) by using a bovine macrophage cell line (BoMacs). Toxicol Appl Pharmacol. 318:33–40. [DOI] [PubMed] [Google Scholar]
- Pomini AM, Ferreira DT, Braz R, Saridakis HO, Schmitz W, Ishikawa NK, Faccione M. 2006. A new method for asperphenamate synthesis and its antimicrobial activity evaluation. Nat Prod Res. 20:537–541. [DOI] [PubMed] [Google Scholar]
- Qi J, Shao CL, Li ZY, Gan LS, Fu XM, Bian WT, Zhao HY, Wang CY. 2013. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. Fungus. J Nat Prod. 76:571–579. [DOI] [PubMed] [Google Scholar]
- Qi QY, Bao L, Ren JW, Han JJ, Zhang ZY, Li Y, Yao YJ, Cao R, Liu HW. 2014. Sterhirsutins A and B, two new heterodimeric sesquiterpenes with a new skeleton from the culture of Stereum hirsutum collected in Tibet Plateau. Org Lett. 16:5092–5095. [DOI] [PubMed] [Google Scholar]
- Qi QY, Ren JW, Sun LW, He LW, Bao L, Yue W, Sun QM, Yao YJ, Yin WB, Liu HW. 2015. Stucturally diverse sesquiterpenes produced by a Chinese Tibet fungus Stereum hirsutum and their cytotoxic and immunosuppressant activities. Org Lett. 17:3098–3101. [DOI] [PubMed] [Google Scholar]
- Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J. 2011. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 77:3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Kusano M, Takeuchi S, Fujioka S, Inokuchi T, Kimura Y. 2002. Aspterric acid and 6-hydroxymellein, inhibitors of pollen development in Arabidopsis thaliana, produced by Aspergillus terreus. Z Naturforsch C Biosci. 57:459–464. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Pil GB, Heo SJ, Lee HS, Lee JS, Lee YJ, Lee J, Won HS. 2016. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar Drugs. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierle DB, Stierle AA, Girtsman T, McIntyre K, Nichols J. 2012. Caspase-1 and −3 inhibiting drimane sesquiterpenoids from the extremophilic fungus Penicillium solitum. J Nat Prod. 75:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YL, Zhang XY, Zheng ZH, Xu XY, Qi SH. 2014. Three new polyketides from marine-derived fungus Penicillium citrinum SCSGAF 0167. Nat Prod Res. 28:239–244. [DOI] [PubMed] [Google Scholar]
- Tang HY, Zhang Q, Li H, Gao JM. 2015. Antimicrobial and allelopathic metabolites produced by Penicillium brasilianum. Nat Prod Res. 29:345–348. [DOI] [PubMed] [Google Scholar]
- Tansakul C, Rukachaisirikul V, Maha A, Kongprapan T, Phongpaichit S, Hutadilok-Towatana N, Borwornwiriyapan K, Sakayaroj J. 2014. A new phenalenone derivative from the soil fungus Penicillium herquei PSU-RSPG93. Nat Prod Res. 28:1718–1724. [DOI] [PubMed] [Google Scholar]
- Wang B, Wang L. 2013. Penicillium kongii, a new terverticillate species isolated from plant leaves in China. Mycologia. 105:1547–1554. [DOI] [PubMed] [Google Scholar]
- Xue J, Wu P, Xu L, Wei X. 2014. Penicillitone, a potent in vitro anti-inflammatory and cytotoxic rearranged sterol with an unusual tetracycle core produced by Penicillium purpurogenum. Org Lett. 16:1518–1521. [DOI] [PubMed] [Google Scholar]
- Yuan L, Li Y, Zou C, Wang C, Gao J, Miao C, Ma E, Sun T. 2012. Synthesis and in vitro antitumor activity of asperphenamate derivatives as autophagy inducer. Bioorg Med Chem Lett. 22:2216–2220. [DOI] [PubMed] [Google Scholar]
- Yuan L, Wang JH, Sun TM. 2010. Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine. Chinese Chem Lett. 21:155–158. [Google Scholar]
- Zhang Z, Guo W, He X, Che Q, Zhu T, Gu Q, Li D. 2016. Peniphenylanes A-G from the deep-sea-derived fungus Penicillium fellutanum HDN14-323. Planta Med. 82:872–876. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Huang GL, Xu Y, Song XM, Yao J, Liu H, Wang RP, Sun XP. 2016. A new benzopyrans derivatives from a mangrove-derived fungus Penicillium citrinum from the South China Sea. Nat Prod Res. 30:821–825. [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen C, Wang J, Li XN, Wei G, Guo Y, Yao G, Luo Z, Zhang J, Xue Y, et al. 2015. Penicamedine A, a highly oxygenated hexacyclic indole alkaloid from Penicillium camemberti. Chem Biodivers. 12:1547–1553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


