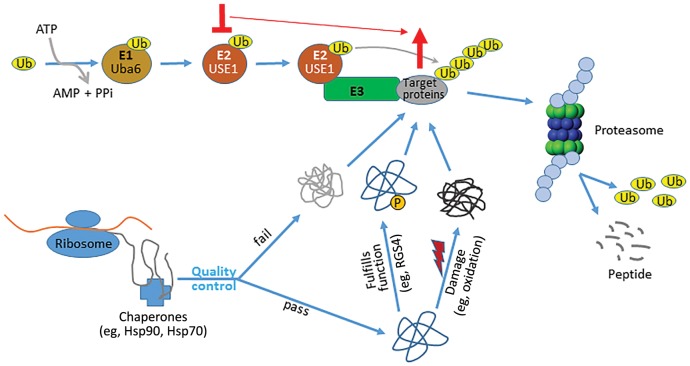
Figure 1.
USE1 is a critical ubiquitin-conjugating enzyme in lung cancer cells. Nascent proteins synthesized at ribosomes undergo folding and maturation with the assistance of chaperones such as Hsp70 and Hsp90. Correctly folded proteins are translocated to other parts of the cell and perform their physiological functions, eg, activated transmembrane GPCRs stimulate signaling by promoting the exchange of GDP with GTP on Gα, while regulator of G-protein signaling (RGS) proteins inhibit Gα signaling by promoting hydrolysis of the bound GTP. These proteins are subsequently marked, such as by phosphorylation, for degradation. Incorrectly folded, or correctly folded but damaged (such as by oxidation), proteins are also degraded to maintain proteostasis. The major protein degradation pathway, ubiquitination, starts with charging the ubiquitin-activating enzyme E1 (eg, Uba6) with a ubiquitin moiety. The ubiquitin is in turn transferred to a ubiquitin-conjugating enzyme E2 (eg, USE1) and subsequently to proteins targeted for degradation via association of the E2 with a ubiquitin-ligase E3. Ubiquitinated proteins are eventually degraded by the proteasome, and the free ubiquitin molecules are recycled. Perturbation of the ubiquitination pathway (eg, knockdown of USE1) disrupts proteostasis and results in accumulation of misfolded and/or damaged proteins, ultimately leading to cell death via apoptosis. In addition to these general effects, functional disruption of a specific component of the pathway, such as USE1, would also cause accumulation of its substrates (eg, RGS4), thus enhancing or prolonging their physiological function (eg, RGS4 as a tumor suppressor).