Abstract
Background: Compelling evidence shows that progestins regulate breast cancer growth. Using preclinical models, we demonstrated that antiprogestins are inhibitory when the level of progesterone receptor isoform A (PR-A) is higher than that of isoform B (PR-B) and that they might stimulate growth when PR-B is predominant. The aims of this study were to investigate ex vivo responses to mifepristone (MFP) in breast carcinomas with different PR isoform ratios and to examine their clinical and molecular characteristics.
Methods: We performed human breast cancer tissue culture assays (n = 36) to evaluate the effect of MFP on cell proliferation. PR isoform expression was determined by immunoblotting (n = 282). Tumors were categorized as PRA-H (PR-A/PR-B ≥ 1.2) or PRB-H (PR-A/PR-B ≤ 0.83). RNA was extracted for Ribo-Zero-Seq sequencing to evaluate differentially expressed genes. Subtypes and risk scores were predicted using the PAM50 gene set, the data analyzed using The Cancer Genome Atlas RNA-seq gene analysis and other publicly available gene expression data. Tissue microarrays were performed using paraffin-embedded tissues (PRA-H n = 53, PRB-H n = 24), and protein expression analyzed by immunohistochemistry. All statistical tests were two-sided.
Results: One hundred sixteen out of 222 (52.3%) PR+ tumors were PRA-H, and 64 (28.8%) PRB-H. Cell proliferation was inhibited by MFP in 19 of 19 tissue cultures from PRA-H tumors. A total of 139 transcripts related to proliferative pathways were differentially expressed in nine PRA-H and seven PRB-H tumors. PRB-H and PRA-H tumors were either luminal B or A phenotypes, respectively (P = .03). PRB-H cases were associated with shorter relapse-free survival (hazard ratio [HR] = 2.70, 95% confidence interval [CI] = 1.71 to 6.20, P = .02) and distant metastasis–free survival (HR = 4.17, 95% CI = 2.18 to 7.97, P < .001). PRB-H tumors showed increased tumor size (P < .001), Ki-67 levels (P < .001), human epidermal growth factor receptor 2 expression (P = .04), high grades (P = .03), and decreased total PR (P = .004) compared with PRA-H tumors. MUC-2 (P < .001) and KRT6A (P = .02) were also overexpressed in PRB-H tumors.
Conclusion: The PRA/PRB ratio is a prognostic and predictive factor for antiprogestin responsiveness in breast cancer.
The progesterone receptor (PR) is a member of the steroid nuclear receptor family. There is increasing clinical (1,2) and experimental evidence (3–5) suggesting its relevance in regulating breast cancer development and growth. However, PR levels are still only used as a surrogate marker for estrogen receptor alpha (ERα) activity (6,7).
Two PR isoforms are transcribed from a single gene (8), and each isoform possesses unique tissue-specific functions (9). PR-A (94 kDa) is entirely contained within the larger PR-B (115 kDa) protein, making them difficult to discriminate by immunohistochemistry (IHC) or polymerase chain reaction (PCR). Few breast cancer studies have evaluated the PR isoforms (10–15). Among those that have, the consensus is that PR-A is the prevailing isoform, although contradictory data exist concerning its prognostic value. Currently, immunoblotting (IB) is the most reproducible method of discriminating between the isoforms to obtain the PR-A/PR-B ratio.
Using medroxyprogesterone acetate (MPA)–induced murine carcinomas and human breast cancer xenografts engineered to express different PR isoform ratios, we demonstrated that only tumors with PR-A levels higher than PR-B levels regress with antiprogestins (16–18). Moreover, constitutive resistant tumors were resensitized to antiprogestin treatment when they re-expressed PR-A after treatment with demethylating agents and HDAC inhibitors (18,19).
Antiprogestins have induced different responses in clinical trials (reviewed in [20–22]). We hypothesized that antiprogestins, together with conventional antiestrogens, offer a valid therapeutic approach for tumors with high PR-A levels. The aim of this study was to investigate the responses of breast cancers with different PR isoform ratios to MFP treatment and to evaluate their molecular portraits and clinical features.
Methods
Patient Tumor Specimens
Tissue samples were obtained from 395 patients undergoing breast cancer surgery from 2007 to 2015. The study was approved by the institutional review boards of the Hospital Rivadavia, Buenos Aires (n = 20), Hospital Magdalena V de Martínez, General Pacheco, Buenos Aires (n = 375), and IBYME-CONICET. Informed consent was obtained from all patients. The samples were flash-frozen and/or kept in tissue culture medium. Patients were excluded when inconsistent data between IB and IHC were obtained or if the quality of an IB was not acceptable.
Primary Cultures and Organotypic Tissue Cultures
Fresh tissues were processed within four hours of resection. Primary cultures were performed as previously described (Supplementary Methods, available online) (23).
Tissue slices (100 µm thick) were obtained using a McIlwain Tissue Chopper (Ted Pella, Inc, Redding, CA) and incubated in DMEM/F-12 without phenol red (Sigma-Aldrich, St. Louis, MO), with 10% fetal calf serum (FCS; Life Technologies, Carlsbad, CA), with or without MFP (Sigma-Aldrich). After 48 hours, tissues were harvested, formalin-fixed, and paraffin-embedded (FFPE) (24). A pathologist (MM) examined morphological integrity and the presence of viable cancer cells by hematoxylin and eosin (H&E) staining. If adequate, Ki-67 was evaluated by IHC (ab15580, Abcam, Cambridge, MA) as previously described (25). The percentage of positive cells was calculated in a minimum of five fragments per treatment. The average number of cells per tumor was 1039 (median = 869, range = 197–3134 cells). An inhibitory/stimulatory effect was registered if a statistically significant difference was recorded (P < .05).
Immunohistochemical Studies and Tissue Arrays
ER and PR expression were extracted from the clinical histories. Human epidermal growth factor receptor 2 (HER2) expression was evaluated by Gabriela Acosta-Haab (Roche Laboratories, Buenos Aires, Argentina). Four tissue microarrays (TMAs), one with 64 samples and the other three with 24 samples each, were constructed using samples segregated by PR isoform content. Anti-MUC-2 (sc-15334; 1:200), anti-FGF-10 (sc-7917; 1:100), and anti-glucocorticoid receptor (GR; sc-1004, 1:150) antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA), anti-CRISP-3 (14847-1-AP; 1:250) from Proteintech Group Inc. (Rosemont, IL), anti-KRT6A (RB-169p; 1:500) from Covance Inc. (Princeton, NJ), and anti-androgen receptor (AR; 5153P, 1:250) from Cell Signaling (Danvers, MA).
Frozen Samples
Frozen samples were pulverized and separated in TRIzol reagent (Life Technologies) for RNA extraction and in DNA digestion buffer and Pierce buffer (Thermo Scientific, Waltham, MA) for IB.
Immunoblotting
T47D breast cancer cells (ATCC, Manassas, VA) were cultured as described (26), and cytosol and nuclear proteins extracted. Proteins were measured using a Lowry protein assay (27); aliquots of standard preparations were stored at −70 °C.
For IB, 100 µg of extracted protein was electrophoresed on an 8% polyacrylamide gel (16). T47D standards were included as positive controls. Anti-PR (PR1294; Dako, Agilent Technologies, Santa Clara, CA; or H190; Santa Cruz) both at 1:500, and anti-ERK (sc-94; Santa Cruz, 1:1000) antibodies were used. Band intensities were densitometrically measured using Image J (National Institutes of Health; Bethesda, MD). ERK was evaluated to control sample quality.
Criteria for Patient Classification According to PR Isoform Ratio
We established a priori that a nuclear PR-A/PR-B ratio of 1.2 or greater is representative of patients with high PR-A (PRA-H), whereas a PR-B/PR-A ratio of 1.2 or greater is representative of patients with high PR-B (PRB-H). Patients with either ratio falling between 1.2 and 0.83 were considered equimolar. This cutoff value was selected based on the 20% variability observed when quantifying PR-A and PR-B using IBs performed with the same samples at different times. Usually, nuclear and cytosol fractions gave coincident values. In borderline cases in which the nuclear value was higher than 1.1 or lower than 0.9 and the cytosolic value was clearly PRA-H or PRB-H, the cytosolic value was considered.
RNA Isolation and RNA-Seq Data Analysis
RNA extracted from 22 samples was processed at the University of North Carolina. Sixteen samples proved suitable for RNA sequencing analysis (Ribo-Zero-Seq); two samples were excluded as outliers. RNA was isolated and purified as described in the Supplementary Methods (available online). Heatmap visualization of differentially expressed transcripts and PAM50 genes was achieved using MultiExperiment Viewer software (MeV v4.9) (28). Intrinsic subtype classification of breast cancer samples was performed using the 50-gene (PAM50) predictor bioclassifier R script (29) and functional enrichment analyses using the ClueGo Cytoscape's plug-in (http://www.cytoscape.org/) and InnateDB resource (http://www.innatedb.com/) based on a list of deregulated transcripts between PRA-H and PRB-H tumors (FDR < 0.05, log2 FC > ±1). To further explore the prognostic value of the PR isoforms in invasive breast carcinomas, we evaluated information from two publicly available gene expression data sets from the UCSC Xena resource (https://xena.ucsc.edu/). We analyzed 586 luminal-like and ER+/PR+ primary invasive breast carcinomas derived from the The Cancer Genome Atlas–Breast Invasive Carcinoma project (30) and 355 from a study reported by Yau et al. (31). Tumor classification in PRA-H and PRB-H cases was performed based on low or high expression levels of PR-associated signatures (eg, PRB-H tumors were defined as having high expression of PRB-H genes and low of PRA-H genes) using the StepMiner one-step algorithm (32) implemented in R. PRA-H and PRB-H cases were then compared.
Statistical Analysis
Absolute and relative frequencies of the main patient characteristics were calculated. To compare the effect of MFP on individual primary/tissue cultures, the Mann Whitney test was used. The chi-square test was used to compare the proportion of PRA-H and PRB-H samples inhibited by MFP. A total of 19 patients per group was predicted to detect statistically significant differences between both groups (80% vs 40%; type I error: 5%, type II error: 10%; https://select-statistics.co.uk/calculators/sample-size-calculator-two-proportions/). The Wilcoxon test was used to evaluate possible associations between ER, AR, and GR expression and MFP responsiveness. To compare clinicopathological parameters among the two groups, the chi-square test and Fisher’s exact test were used for categorical variables. The Student’s t test was used to compare the mean age between the two groups. The Cochran-Armitage trend test was used to evaluate possible trends in histologic grade, PR status, and ER status. Log-rank tests were used to analyze Kaplan-Meier curves using the Survival R package. A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided.
Results
Patient Distribution According to PR Isoform Ratio
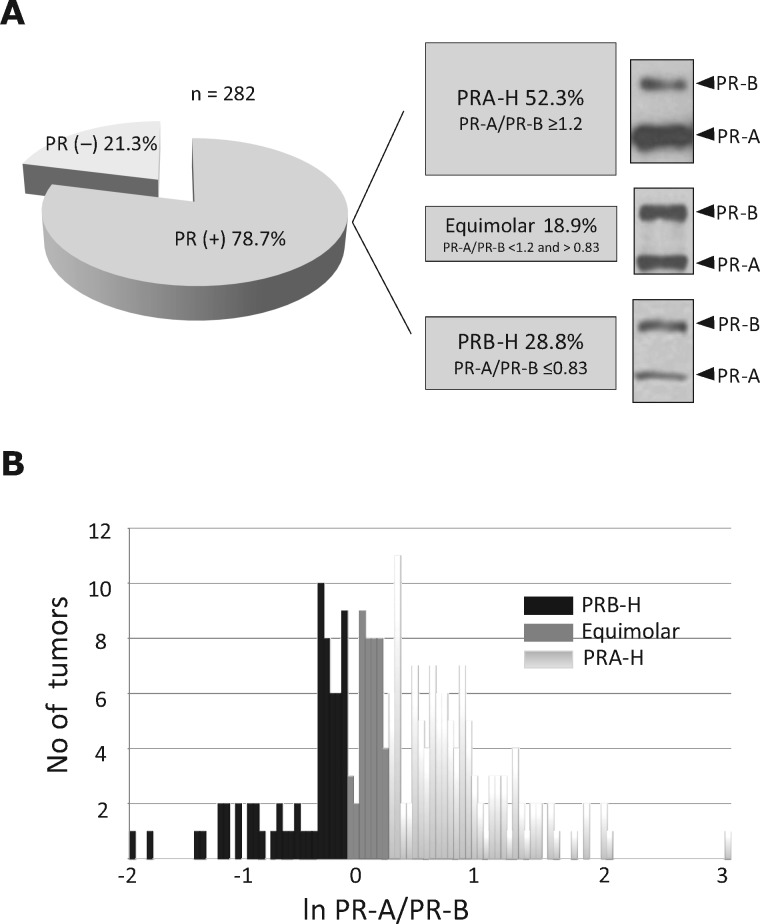
In total, 282 samples were included; patient and tumor features are shown in Table 1. The median PR-A/PR-B ratio across all PR+ samples was 1.2 (range = 0.1–20.2, 25.0% percentile: 0.825, 75.0% percentile: 2, 95% confidence interval [CI] = 1.48 to 1.94). Of the 222 PR+ breast cancers, 116 were PRA-H predominant (52.3%), 64 were PRB-H predominant (28.8 %), and 42 were equimolar (Figure 1A). Figure 1B illustrates the frequency distribution of the PR-A/PR-B ratio.
Table 1.
Clinicopathological parameters of patients*
| Characteristics | No. (%) |
|---|---|
| Age (n = 247), y | |
| <40 | 13 (5.3) |
| 40–50 | 50 (18.2) |
| 51–60 | 57 (23.1) |
| >60 | 127 (51.4) |
| Tumor size, cm (n = 202) | |
| <2 | 37 (18.3) |
| 2–5 | 137 (67.8) |
| >5 | 28 (13.9) |
| Histologic grade (n = 247) | |
| I | 47 (19.0) |
| II | 71 (28.7) |
| III | 129 (52.2) |
| PR-positive (n = 282) | 222 (78.7) |
| ERα-positive (n = 234) | 197 (84.2) |
| HER2-positive (n = 222) | 40 (18.0) |
| Triple-negative (n = 239) | 17 (7.1) |
ERα = estrogen receptor alpha; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
Figure 1.
Classification of breast tumors according to their progesterone receptor isoform A (PR-A)/progesterone receptor isoform B (PR-B) ratios. A) Left: Diagram showing the percentage of PR+ tumors. Right: The PR isoform ratio was evaluated densitometrically measuring the band intensity of each isoform in immunoblots. PR+ tumors were classified into three categories according to the PR-A/PR-B ratio: PRA-H (52.3%), equimolar (18.3%), and PRB-H (28.8%). A representative immunoblot of each category is shown. B) Frequency diagram showing the distribution of the PR-A/PR-B ratio for all evaluated PR+ tumors. PR = progesterone receptor; PR-A = PR isoform A; PR-B = PR isoform B; PRA-H = tumors with higher levels of PR-A than PR-B; PRB-H = tumors with higher levels of PR-B than PR-A.
Effect of MFP in Cultures Of Breast Cancer Samples Classified According Their PR-A/PR-B Ratio
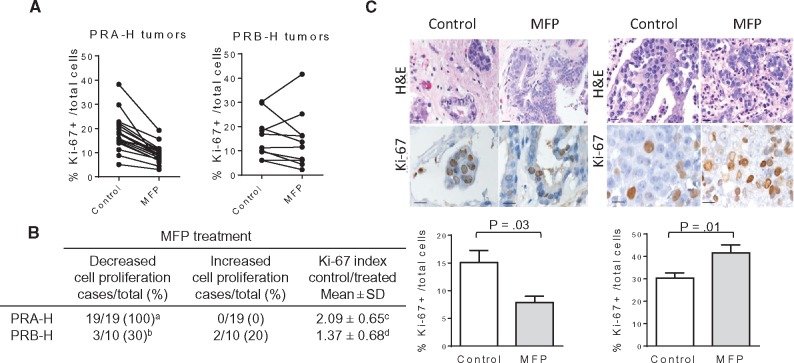
From 70 primary culture attempts, successful subcultures were achieved in only nine cases (13% efficiency). In four out of five of the PRA-H samples, MFP decreased the number of cells (P = .03) (Supplementary Figure 1, available online); different responses were observed in non-PRA-H samples. Considering the low efficiency of successful subculture, we explored a novel tissue culture method (described in the “Methods” section). Using this methodology, 36 of 112 cases (32.1%) were successful, increasing the efficiency previously obtained with primary cultures. In vitro studies were performed by researchers blinded to the IB results. Statistically significant inhibition of Ki-67 expression was observed after 48 hours of MFP treatment in 19 of 19 of the PRA-H samples and three of 10 of the PRB-H samples (Figure 2A). MFP stimulated Ki-67 expression in two of 10 of the PRB-H samples (Figure 2, A and B). In total, one of four of the equimolar and one of three of the PR-negative samples were inhibited by MFP (data not shown). Figure 2C shows two representative examples of Ki-67 staining. Overall, the tissue cultures maintained the morphological features of their parental tumors (Supplementary Figure 2, available online). Supplementary Table 1 (available online) shows the individual characteristics of the tumors that were included as well as their ERα, PR, AR, and GR status. Collectively, the results from these in vitro studies demonstrate that PRA-H tumors were consistently inhibited by MFP, whereas PRB-H, equimolar, or PR-negative tumors were only occasionally inhibited. Most importantly, MFP may stimulate cell proliferation in some PRB-H samples. ERα (P = .93), AR (P = .18), and GR (P = .82) status were not associated with MFP responsiveness.
Figure 2.
Effects of mifepristone (MFP) in tissue cultures of breast cancer samples classified according their progesterone receptor isoform A (PR-A)/progesterone receptor isoform B (PR-B) ratio. A)Proliferative responses (Ki-67 staining) of primary breast cancer tissues cultured ex vivo with or without 10 nM MFP for 48 hours. The percentage of cells with nuclear Ki-67 staining with respect to all tumor cells was calculated in at least five different sections per experimental group. The mean values were plotted and connected with a line. A statistically significant decrease in Ki-67 staining was observed in each of the 19 cultures tested (P < .001, two-sided paired t test), whereas variable responses were obtained in 10 PRB-H cases (P = .54). B) Table illustrating the number of tumors that were inhibited or stimulated by MFP according to the PR-A/PR-B ratio; a vs b: P < .001, two-sided Fisher’s exact test; c vs d: P = .01, two-sided Student’s t test. C) Two representative cases of PRA-H (left) and PRB-H (right) tissue cultures are shown. Top: Hematoxylin and eosin images of paraffin-embedded tissue cultures and Ki-67 immunohistochemistry showing nuclear staining; bar = 50 μm. Bottom: Quantification of Ki-67+ cells/all tumor cells in five different explants of the examples shown in (B); P values were calculated using the two-sided Mann Whitney test. H&E = hematoxylin and eosin; MFP= mifepristone; PR = progesterone receptor; PR-A = PR isoform A; PR-B = PR isoform B; PRA-H = tumors with higher levels of PR-A than PR-B; PRB-H = tumors with higher levels of PR-B than PR-A.
Transcriptome Analysis of PRB-H and PRA-H Samples
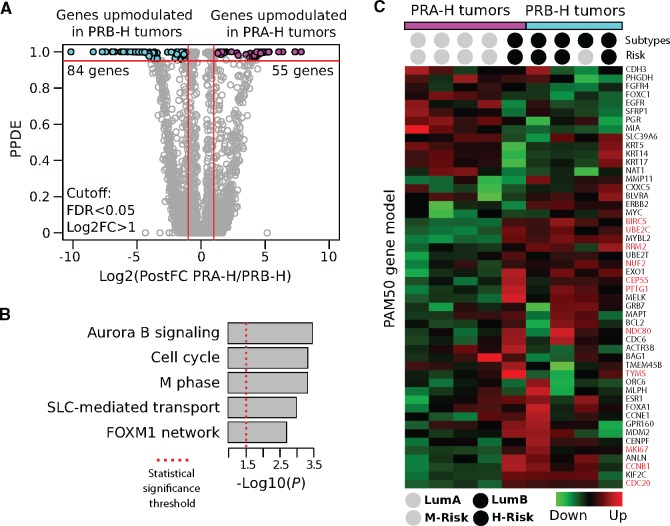
RNA-seq analysis of nine PRA-H and seven PRB-H tumors revealed 139 genes that were differentially expressed (FDR < 0.05, Log2 FC > 1): 84 were upregulated in the PRB- H tumors (and downregulated in PRA-H), while 55 were upregulated in the PRA-H tumors (Figure 3A;Supplementary Table 2, available online). Pathway enrichment analysis of the deregulated transcripts revealed that they were related to specific bioprocesses associated with the cell proliferation signature of breast cancer cells, including the Aurora B (P < .001) and FOXM1 (P = .004) signaling pathways, M phase (P < .001), and other cell cycle (P < .001) processes (Figure 3B).
Figure 3.
Transcriptome analysis of invasive breast carcinomas according to progesterone receptor (PR) isoforms. A) Volcano-like plot of differentially expressed genes among the tumors with higher levels of isoform A of progesterone receptor (PR-A) than isoform B (PR-B; PRA-H) and tumors with higher levels of PR-B than PR-A (PRB-H) breast carcinomas (false discovery rate [FDR] < 0.05, fold-change > 2). The x-axis represents the posterior fold-changes in the expression levels measured in PRA-H vs PRB-H tumors, and the y-axis represents the posterior probability of differential expression (posterior probabilities of being differentially expressed > 0.95 ≥ FDR < 0.05). B) Functional enrichment analysis of the differentially expressed genes between the PRA-H and PRB-H breast carcinomas. C) Prediction of breast cancer intrinsic subtypes using the PAM50 gene model based on RNA-seq profiles. Tumors were denominated as high risk (H-Risk) or moderate risk (M-Risk) based on the intrinsic subtype assigned and on their proliferative score (Risk of recurrence P value). Proliferation-related genes are highlighted in red. All statistical tests were two-sided. FDR = false discovery rate; Log2FC = logarithm2 fold-change; LumA = luminal A; LumB = luminal B; M phase = mitotic phase; PPDE = posterior probabilities of being differentially expressed; PR = progesterone receptor; PR-A = PR isoform A; PR-B = PR isoform B; PRA-H = tumors with higher levels of PR-A than PR-B; PRB-H = tumors with higher levels of PR-B than PR-A; SLC-mediated transport = solute-carrier gene-mediated transport.
Using the PAM50 gene set to analyze gene expression, we observed that the genes overexpressed in the PRB-H tumors were 1) highly concentrated within the group of genes that characterize the luminal B subtype and 2) proliferation-related genes (Figure 3C). Based on this expression pattern, these tumors could be classified as high risk. In contrast, the PRA-H tumors were associated with the luminal A subtype, which indicates they were of a lower risk.
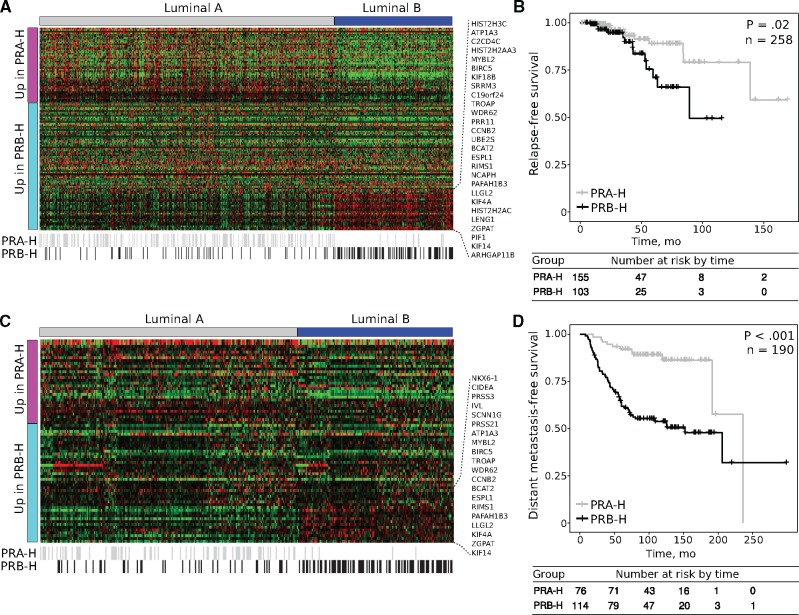
Further analysis of the deregulated transcripts discriminating the PRA-H tumors from the PRB-H tumors among two independent luminal-like/ER- and PR-positive breast cancer data sets suggested that PRB-H patients have a worse prognosis and are more likely to have luminal B carcinomas, whereas PRA-H patients are more likely to have the luminal A intrinsic subtype (P = .03) (Figure 4, A and C). Survival analysis revealed that PRB-H cases are associated with shorter relapse-free survival (hazard ratio [HR] = 2.70, 95% CI = 1.71 to 6.20, P =.02) (Figure 4B) and distant metastasis–free survival (HR = 4.17, 95% CI = 2.18 to 7.97, P < .001) (Figure 4D) compared with their PRA-H counterparts.
Figure 4.
Comparative analysis of tumors with higher levels of progesterone receptor isoform A (PR-A) than progesterone receptor isoform B (PR-B; PRA-H) and tumors with higher levels of PR-B than PR-A (PRB-H) gene expression signatures and the prognostic value of predicted PR isoforms across two independent breast cancer data sets. A) Heatmap of PRA-H- and PRB-H-associated gene expression signatures among 586 luminal-like and PR+ primary invasive breast carcinomas obtained from the The Cancer Genome Atlas (TCGA)–Breast Invasive Carcinoma project. Black lines at the bottom indicate the predicted PR isoforms. B) Relapse-free survival curves of the predicted PRA-H and PRB-H cases among the TCGA data set. C) Heatmap of PRA-H- and PRB-H-associated gene expression signatures among 355 luminal-like primary invasive breast carcinomas obtained from a data set reported by Yau et al. in 2010. Black lines at the bottom indicate the predicted PR isoforms. D) Distant metastasis-free survival curves of the predicted PRA-H and PRB-H cases among the same data set. P values were calculated using two-sided log-rank test. PR = progesterone receptor; PR-A = PR isoform A; PR-B = PR isoform B; PRA-H = tumors with higher levels of PR-A than PR-B; PRB-H = tumors with higher levels of PR-B than PR-A.
KRT6A and MUC-2 Expression in PRA-H and PRB-H Samples
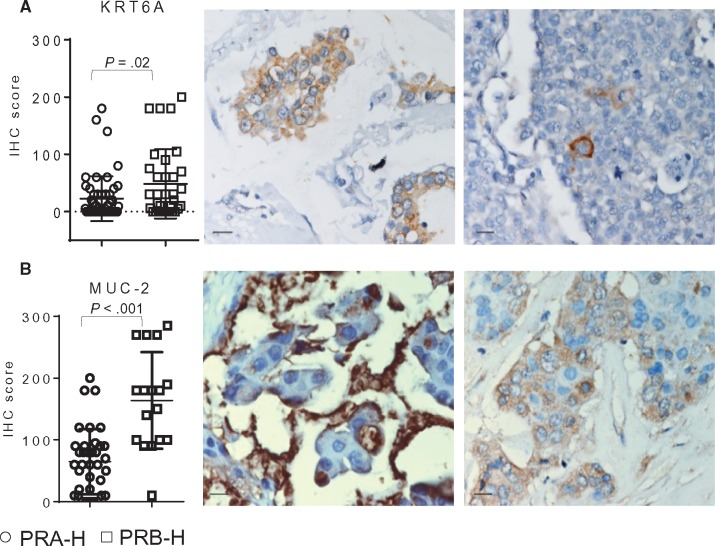
Four of the deregulated genes were selected for protein expression evaluation by IHC in TMA performed using both PRA-H and PRB-H samples. Transcription levels of KRT6A (P = .02) and MUC-2 (P < .001), upregulated in PRB-H, were corroborated at the protein level by IHC (Figure 5, A and B). No statistically significant differences were found regarding CRISP-3 (upregulated in PRB-H) or FGF-10 (upregulated in PRA-H) expression. Intense KRT6A expression was observed in isolated cells mainly in the PRA-H tumors (Figure 5A); a more uniform cytoplasmic staining prevailed in the PRB-H tumors.
Figure 5.
KRT6A and MUC-2 expression in progesterone receptor isoform A (PR-A) than progesterone receptor isoform B (PR-B; PRA-H) and tumors with higher levels of PR-B than PR-A (PRB-H) samples. Protein expression levels for two out of four genes selected from a list of 139 deregulated genes identified in RNA-seq studies. Protein expression was studied by immunohistochemistry (IHC) in TMAs constructed using PRA-H (n = 53) and PRB-H (n = 24) samples. A score was calculated to represent the percentage of stained cells and the intensity of the staining (0–3). A) KRT6A was upregulated in the transcriptome analysis of PRB-H samples. IHC studies confirmed a corresponding increase in protein expression (P = .02). Images illustrate the heterogeneous cytoplasmic staining observed (left). Occasionally, single cells were intensively stained (right). B) MUC-2 was also upregulated in the transcriptome analysis of PRB-H samples, and IHC studies confirmed a corresponding increase in MUC-2 protein expression (P < .001). Images illustrate the typical MUC-2 intense staining pattern observed in a mucinous area (left) as well as the representative cytoplasmic staining observed (right). Scale bar = 50 µm. Error bars represent SD. P values were calculated using two-sided Mann Whitney test. IHC = immunohistochemistry; PR = progesterone receptor; PR-A = PR isoform A; PR-B = PR isoform B; PRA-H = tumors with higher levels of PR-A than PR-B; PRB-H: tumors with higher levels of PR-B than PR-A.
Clinical Features of PRA-H and PRB-H Patients
No statistically significant differences were found between the PRA-H and PRB-H patients in average age, although the latter had a mean age that was three years younger than the former. Compared with the PRA-H patients, at the time of surgery, the PRB-H patients had larger tumors (P < .001) with worse histological grade (P = .03) and lower levels of total PR (P = .004). No differences or trends were observed regarding ERα status, number of positive lymph nodes, or distant metastases. Positive HER2 expression (P = .04) and high Ki-67 expression (P < .001) prevailed in the PRB-H tumors (Table 2). Both are biomarkers of worse prognosis.
Table 2.
Clinicopathological parameters of patients by isoform PR status
| Clinopathological parameter | PRA-H No. (%) | PRB-H No. (%) | P |
|---|---|---|---|
| PR+ patients | 116/180 (64.4) |
64/180 (35.6) |
<.001* |
| Age, mean ± SD, y | 63 ±14.1 | 60 ± 12.9 | .21† |
| Tumor size, cm | <.001‡ | ||
| Nonpalpable | 1/99 (1.0) | 0/55 (0.0) | |
| <2 | 48/99 (48.5) | 9/55 (16.4) | |
| 2–5 | 42/99 (42.4) | 34/55 (61.8) | |
| >5 | 8/99 (8.1) | 12/55 (21.8) | |
| Positive nodes | 49/92 (53.3) | 19/37 (51.4) | .84* |
| Positive metastases | 4/66 (6.1) | 1/38 (2.6) | .65‡ |
| Histologic type | .51* | ||
| IC NST | 91/112 (81.3) | 47/58 (81.0) | |
| ILC | 14/112 (12.5) | 5/58 (8.6) | |
| SS | 7/112 (6.3) | 6/58 (10.3) | |
| Histologic grade | .01§ | ||
| I | 9/88 (10.2) | 1/46 (2.2) | |
| II | 35/88 (39.7) | 12/46 (26.0) | |
| III | 44/88 (50.0) | 33/46 (71.7) | |
| PR status | .004§ | ||
| 0 | 0/108 (0.0) | 0/52 (0.0) | |
| 1–25 | 22/108 (20.4) | 18/52 (34.6) | |
| 26–50 | 11/108 (10.2) | 13/52 (25.0) | |
| 51–75 | 22/108 (20.4) | 4/52 (7.7) | |
| 76–100 | 53/108 (49.1) | 17/52 (32.7) | |
| ERα status | .23§ | ||
| 0 | 1/107 (0.9) | 1/52 (1.9) | |
| 1–25 | 12/107 (11.2) | 7/52 (13.5) | |
| 26–50 | 4/107 (3.7) | 4/52 (7.7) | |
| 51–75 | 19/107 (17.8) | 11/52 (21.2) | |
| 76–100 | 71/107 (66.4) | 29/52 (55.8) | |
| HER2-positive | 6/102 (5.9) | 8/50 (16.0) | .04* |
| Ki-67 status (<14%) | 43/58 (74.1) | 7/23 (30.4) | <.001* |
P value was calculated using a two-sided chi-square test. ERα = estrogen receptor alpha; IC NST = invasive carcinoma of no special type; ILC = invasive lobular carcinoma; PR = progesterone receptor; PRA-H = tumors with higher levels of isoform A of progesterone receptor (PR-A) than isoform B (PR-B); PRB-H = tumors with higher levels of PR-B than PR-A; SS = special subtype.
P value was calculated using a two-sided Student’s t test
P value was calculated using a two-sided Fisher exact test.
P value was calculated using a two-sided Cochran-Armitage trend test.
Discussion
In this study, we showed that PRA-H breast cancers can be inhibited by MFP ex vivo and that the PR-A/PR-B ratio serves as a prognostic factor, with PRB-H patients having a worse prognosis. Our study emphasizes the relevance of determining the PR isoform ratio before starting antiprogestin treatments. Of the two clinical trials currently evaluating antiprogestins for PR+ breast cancer—trial NCT02052128 is assessing onapristone and trial NCT01800422 is assessing telapristone acetate—neither is considering the PR isoform ratio as a possible inclusion criterion. On the basis of the data reported herein, we have recently started a trial evaluating the use of neo-adjuvant MFP treatment for patients with PRA-H breast cancer (NCT02651844; MIPRA).
The prognostic value of PR isoforms in breast cancer remains controversial, and this has been extensively discussed in recent reviews (33,34). Isoform expression has been evaluated by IB in few other studies (10,11,13). Bamberger et al. (11) and Hopp et al. (13) suggested that high PR-A levels potentially correlate with worse prognosis or recurrence after tamoxifen treatment. Our study, which focused on the PR-A/PR-B ratio rather than on absolute PR isoform values, indicates that patients with lower PR-A levels than PR-B levels may have a worse prognosis. Similar results associating PR-A silencing due to promoter methylation with worse prognosis have been communicated by Pathiraja et al. (14). Whereas the previously mentioned studies used cytosolic or total extracts to perform IB, ours is the only study that used nuclear extracts to define the PR-A/PR-B isoform ratio. Moreover, the conditions we used to define a tumor as PRA-H or PRB-H are considerably more stringent than those used in previous studies (11,13).
Immunofluorescence (IF), IHC, and/or PCR have been used to define the PR-A/PR-B ratio in two breast cancer studies (12,15). Different antibodies have been reported to specifically recognize PR-A in FFPE tissues (15,35); however, the measurement of a ratio of intensities using different antibodies with different affinities may not be as accurate as the use of IB. Similarly, in PCR studies, PR-A values are obtained by subtracting values obtained using primers that recognize both PR isoforms and those recognizing only PR-B (12,36–39). A correlation between the protein and mRNA expression of PR isoforms has not yet been proven using large numbers of tumors. Mote et al. (15) used IF to quantity PR isoforms and showed that exogenous hormone replacement therapy results in higher levels of PR-B relative to PR-A and identified a correlation between the PR-A/PR-B ratio and recurrence in patients treated with tamoxifen, but not in those treated with the combination of tamoxifen/aromatase inhibitors. Thus, they proposed that measurements of PR isoforms may help identify the most appropriate ER-targeted therapy.
In vitro studies using cells genetically modified to express different levels of PR-A and/or PR-B have associated PR-A with a gene expression signature that indicates aggressiveness (40,41). However, when the modified cells were transplanted into immunosuppressed mice, they did not behave as expected. Interestingly, T47D-YA tumors expressing PR-A showed a slower growth rate than T47D-YB tumors expressing PR-B, and only the former were statistically significantly inhibited by tamoxifen (42) or MFP (18). These results are concordant with our current data and also with data obtained in preclinical murine, human, and canine models (17–19,43,44).
This is the first study to perform ex vivo evaluations of the effect of antiprogestins in human breast cancers categorized according to the prevailing PR isoforms expressed. Initially, we adapted a tissue culture method used in the field of neurobiology (45); however, a similar method was recently reported for breast cancer samples (46). The effect of MFP on PRA-H tumors was consistent over all 19 samples studied, which implied that our decision to stop the analysis was warranted, even though we only achieved success with 10 PRB-H tumors. Even if the nine remaining samples would have been inhibited by MFP, the difference would have still reached the predicted statistical significance. A stimulatory effect of MFP was detected in two PRB-H samples. Interestingly, the second sample was a relapse from the first, reinforcing the reliability of the tissue culture method utilized. Low concentrations of MFP were used to reduce the drug’s anti-glucocorticoid (47) and anti-androgenic effects (48). Although no association was found between ERα, GR, and AR expression and MFP responsiveness in the PRA-H tumors, a larger number of PRB-H, equimolar, or PR-negative samples must be evaluated to determine whether GR and AR contribute to the inhibitory effect of MFP observed in selected cases.
Molecular and clinical features were concordant, and tumors with higher levels of PR-A than PR-B were associated with biomarkers of better prognosis and a luminal A phenotype. One limitation of our study is that we included all patients who underwent surgical tumor resection, regardless of tumor stage. Further validation with independent and larger cohorts of patients with different tumor stages is warranted. When we divided our cohort of luminal patients into phenotype A or B according to the Ki-67 expression, there was a statistically significant association between the luminal A phenotype and the PRA-H status (not shown). However, this association was not strong enough to exclude all PRB-H tumors. Data-mining results supported these findings. These results emphasize the need to identify alternative biomarkers that may help categorize PRA-H and PRB-H tumors for cases in which IB cannot be performed.
According to RNA sequencing data, MUC-2 and KRT6A were highly expressed in PRB-H tumors, and these results were confirmed by IHC. MUC-2 is exclusively expressed in breast cancer and not in normal cells (49); KRT6A is highly expressed in basal tumors (50) and in progenitor mammary cells (51). Studies are now being performed to develop an IHC algorithm that includes assessments of Ki-67, HER2, MUC-2, and KRT6A expression as well as other factors such as tumor size, differentiation grade, molecular subtype, and total PR to define a PRA-H or PRB-H phenotype without the need of IB.
Preclinical data suggest that combined targeting of ER and PR improves the effectiveness of single endocrine treatments (52). If this is the case, adjuvancy seems the best scenario for combined therapies using antiprogestins. It has recently been demonstrated that progestins may induce ER rewiring, thus inhibiting estrogen-induced cell proliferation (53). Standard endocrine therapies involve the use of tamoxifen, aromatase inhibitors or fulvestrant, resulting in the better positioning of antiprogestins than progestins for the treatment of PRA-H patients. Ongoing studies are expected to provide data supporting if progestins are better options for tumors with low PRA/PRB ratios.
In summary, in this study we provided strong evidence that antiprogestins should be a therapeutic option for luminal breast cancers with higher levels of PR-A than PR-B.
Funding
This work was supported by Agencia Nacional de Promoción de Ciencia y Tecnología (ANAPCYT; PICT2007/932, PICT 2012/1091), CONICET: PIP 2013-16, Fundación Roemmers, Fundación Gador, Fundación Baron, and Fundación Fiorini. Fundación Sales supported the study until March 2014. GS and MM are CONICET fellows, and PR, MCA, and CL are members of the Research Career; AE and MA are fellows of Instituto Nacional de Cancer (INC). CMP was supported by funds from the National Cancer Institute (NCI) Breast Specialized Programs of Research Excellence (SPORE) program (P50-CA58223-09A1) and by the Breast Cancer Research Foundation. A. Molinolo is supported at 25% effort from the Cancer Center Support Grants as the Director of Biorepository and Tissue Technology Shared Resource (3 P30 CA023100 [Lippman, Scott], May 1, 2014, to April 30, 2019).
Notes
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We wish to thank Dr. José Belizán from the Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, for his help with data analysis. We also thank Dr. Marcos Liguori for his comments, Dr. Ana Bagnati from the Hospital Rivadavia for providing 20 samples in 2007, Dr. Alejandro de Nicola and Dr. Florencia Labombarda for generously sharing their McIlwain Tissue Chopper, and Dr. Gabriela Acosta Haab and Roche Laboratories for measuring human epidermal growth factor receptor 2.
Supplementary Material
References
- 1. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;2883:321–333. [DOI] [PubMed] [Google Scholar]
- 2. Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;3629382:419–427. [DOI] [PubMed] [Google Scholar]
- 3. Aupperlee MD, Smith KT, Kariagina A, Haslam SZ.. Progesterone receptor isoforms A and B: Temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;1468:3577–3588. [DOI] [PubMed] [Google Scholar]
- 4. Lange CA, Sartorius CA, Abdel-Hafiz H, Spillman MA, Horwitz KB, Jacobsen BM.. Progesterone receptor action: Translating studies in breast cancer models to clinical insights. Adv Exp Med Biol. 2008;630:94–111. [PubMed] [Google Scholar]
- 5. Lanari C, Lamb CA, Fabris VT, et al. The MPA mouse breast cancer model: Evidence for a role of progesterone receptors in breast cancer. Endocr Relat Cancer. 2009;162:333–350. [DOI] [PubMed] [Google Scholar]
- 6. Nardulli AM, Greene GL, O'Malley BW, Katzenellenbogen BS.. Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: Analysis of estrogen's effect on progesterone receptor synthesis and degradation. Endocrinology. 1988;1223:935–944. [DOI] [PubMed] [Google Scholar]
- 7. Horwitz KB, McGuire WL.. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978;2537:2223–2228. [PubMed] [Google Scholar]
- 8. Giangrande PH, McDonnell DP.. The A and B isoforms of the human progesterone receptor: Two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. [PubMed] [Google Scholar]
- 9. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM.. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;10017:9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham JD, Yeates C, Balleine RL, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;5521:5063–5068. [PubMed] [Google Scholar]
- 11. Bamberger AM, Milde-Langosch K, Schulte HM, Löning T.. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: Correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res. 2000;541:32–37. [DOI] [PubMed] [Google Scholar]
- 12. Ariga N, Suzuki T, Moriya T, et al. Progesterone receptor A and B isoforms in the human breast and its disorders. Jpn J Cancer Res. 2001;923:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopp TA, Weiss HL, Hilsenbeck SG, et al. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;108:2751–2760. [DOI] [PubMed] [Google Scholar]
- 14. Pathiraja TN, Shetty PB, Jelinek J, et al. Progesterone receptor isoform-specific promoter methylation: Association of PRA promoter methylation with worse outcome in breast cancer patients. Clin Cancer Res. 2011;1712:4177–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mote PA, Gompel A, Howe C, et al. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res Treat. 2015;1512:309–318. [DOI] [PubMed] [Google Scholar]
- 16. Helguero LA, Viegas M, Asaithamby A, Shyamala G, Lanari C, Molinolo AA.. Progesterone receptor expression in medroxyprogesterone acetate-induced murine mammary carcinomas and response to endocrine treatment. Breast Cancer Res Treat. 2003;793:379–390. [DOI] [PubMed] [Google Scholar]
- 17. Wargon V, Helguero LA, Bolado J, et al. Reversal of antiprogestin resistance and progesterone receptor isoform ratio in acquired resistant mammary carcinomas. Breast Cancer Res Treat. 2009;1163:449–460. [DOI] [PubMed] [Google Scholar]
- 18. Wargon V, Riggio M, Giulianelli S, et al. Progestin and antiprogestin responsiveness in breast cancer is driven by the PRA/PRB ratio via AIB1 or SMRT recruitment to the CCND1 and MYC promoters. Int J Cancer. 2015;13611:2680–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wargon V, Fernandez SV, Goin M, Giulianelli S, Russo J, Lanari C.. Hypermethylation of the progesterone receptor A in constitutive antiprogestin-resistant mouse mammary carcinomas. Breast Cancer Res Treat. 2011;1262:319–332. [DOI] [PubMed] [Google Scholar]
- 20. Klijn JG, Setyono-Han B, Foekens JA.. Progesterone antagonists and progesterone receptor modulators in the treatment of breast cancer. Steroids. 2000;65(10–11):825–830. [DOI] [PubMed] [Google Scholar]
- 21. Lanari C, Wargon V, Rojas P, Molinolo AA.. Antiprogestins in breast cancer treatment: Are we ready? Endocr Relat Cancer. 2012;193:R35–R50. [DOI] [PubMed] [Google Scholar]
- 22. Goyeneche AA, Telleria CM.. Antiprogestins in gynecological diseases. Reproduction. 2015;1491:R15–R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vázquez SM, Mladovan A, Garbovesky C, Baldi A, Lüthy IA.. Three novel hormone-responsive cell lines derived from primary human breast carcinomas: Functional characterization. J Cell Physiol. 2004;1993:460–469. [DOI] [PubMed] [Google Scholar]
- 24. Vaira V, Fedele G, Pyne S, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S A. 2010;10718:8352–8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vanzulli S, Efeyan A, Benavides F, et al. p21, p27 and p53 in estrogen and antiprogestin-induced tumor regression of experimental mouse mammary ductal carcinomas. Carcinog. 2002;235:749–757. [DOI] [PubMed] [Google Scholar]
- 26. Cerliani JP, Guillardoy T, Giulianelli S, et al. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. 2011;7110:3720–3731. [DOI] [PubMed] [Google Scholar]
- 27. Lowry OH, Rosebrough NJ, Farr AL.. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28. Saeed AI, Sharov V, White J, et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques. 2003;342:374–378. [DOI] [PubMed] [Google Scholar]
- 29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;278:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;4907418:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yau C, Esserman L, Moore DH, Waldman F, Sninsky J, Benz CC.. A multigene predictor of metastatic outcome in early stage hormone receptor-negative and triple-negative breast cancer. Breast Cancer Res. 2010;125:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sahoo D, Dill DL, Tibshirani R, Plevritis SK.. Extracting binary signals from microarray time-course data. Nucleic Acids Res. 2007;3511:3705–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA.. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54:R31–R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdel-Hafiz HA, Horwitz KB.. Role of epigenetic modifications in luminal breast cancer. Epigenomics. 2015;75:847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL.. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;548:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar NS, Richer J, Owen G, Litman E, Horwitz KB, Leslie KK.. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: Implications for unopposed estrogen action. Cancer Res. 1998;589:1860–1865. [PubMed] [Google Scholar]
- 37. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE.. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;858:2897–2902. [DOI] [PubMed] [Google Scholar]
- 38. Turgeon JL, Waring DW.. Differential expression and regulation of progesterone receptor isoforms in rat and mouse pituitary cells and LbetaT2 gonadotropes. J Endocrinol. 2006;1903:837–846. [DOI] [PubMed] [Google Scholar]
- 39. Ke W, Chen C, Luo H, et al. Histone deacetylase 1 regulates the expression of progesterone receptor A during human parturition by occupying the progesterone receptor A promoter. Reprodsci. 2016;237:955–964. [DOI] [PubMed] [Google Scholar]
- 40. Khan JA, Bellance C, Guiochon-Mantel A, Lombès M, Loosfelt H.. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLOS ONE. 2012;79:e45993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mote PA, Graham JD, Clarke CL.. Progesterone receptor isoforms in normal and malignant breast. Ernst Schering Found Symp Proc. 2007;11:77–107. [PubMed] [Google Scholar]
- 42. Sartorius CA, Shen T, Horwitz KB.. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat. 2003;793:287–299. [DOI] [PubMed] [Google Scholar]
- 43. Esber N, Le Billan F, Resche-Rigon M, Loosfelt H, Lombès M, Chabbert-Buffet N.. Ulipristal acetate inhibits progesterone receptor isoform A-Mediated human breast cancer proliferation and BCl2-L1 expression. PLOS ONE. 2015;1010:e0140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guil-Luna S, Stenvang J, Brünner N, et al. Progesterone receptor isoform A may regulate the effects of neoadjuvant aglepristone in canine mammary carcinoma. BMC Vet Res. 2014;10:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Labombarda F, Ghoumari AM, Liere P, De Nicola AF, Schumacher M, Guennoun R.. Neuroprotection by steroids after neurotrauma in organotypic spinal cord cultures: A key role for progesterone receptors and steroidal modulators of GABA(A) receptors. Neuropharmacology. 2013;71:46–55. [DOI] [PubMed] [Google Scholar]
- 46. Faversani A, Vaira V, Moro GP, et al. Survivin family proteins as novel molecular determinants of doxorubicin resistance in organotypic human breast tumors. Breast Cancer Res. 2014;163:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE.. RU 486: A steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci U S A. 1984;8112:3879–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song LN, Coghlan M, Gelmann EP.. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol Endocrinol. 2004;181:70–85. [DOI] [PubMed] [Google Scholar]
- 49. Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK.. Mucins in the pathogenesis of breast cancer: Implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;18152:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;4066797:747–752. [DOI] [PubMed] [Google Scholar]
- 51. Bu W, Chen J, Morrison GD, et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene. 2011;3043:4399–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singhal H, Greene ME, Tarulli G, et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;26:e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;5237560:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.