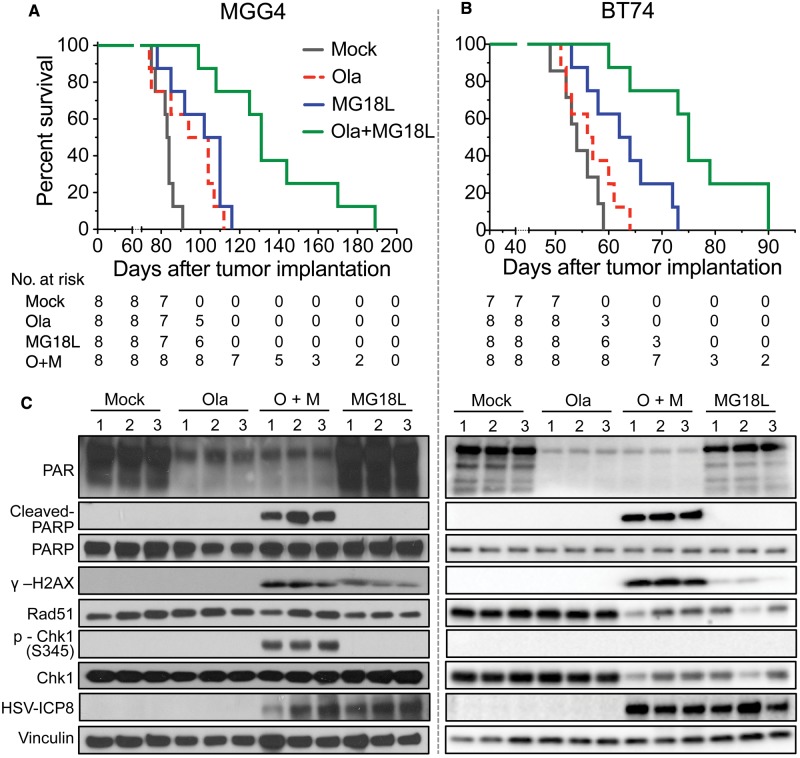
Figure 7.
Efficacy of combination therapy in glioblastoma stem cell–derived intracerebral tumors. A) Mice implanted intracerebrally with 2 x 105 MGG4 cells were treated with olaparib (Ola; 50 mg/kg) in 10% DMSO/10% 2-hydroxyl-propyl-β-cyclodextrine/PBS or vehicle, administered intraperitoneally starting on day 17 postimplantation with six cycles of five-day on and two-day off, and MG18L (1 X 106 pfu) or PBS intratumorally injected on day 19. Mock vs olaparib (P = .03) or vs MG18L (P = .003); olaparib + MG18L vs MG18L (P = .005) or vs olaparib (P = .001); log-rank test. B) Mice implanted intracerebrally with 1 x 105 BT74 cells were treated with olaparib or vehicle administered intraperitoneally daily for 26 days starting on day 9 postimplantation, and MG18L or PBS intratumorally injected on day 11. Mock vs MG18L (P = .009); olaparib + MG18L vs MG18L (P = .005) or vs olaparib (P = .0004); log-rank test. C) DNA damage responses induced by olaparib (Ola), MG18L, or combination (O + M) in vivo. Intracerebral tumors were established with MGG4 (left) and treated with olaparib (Ola) or vehicle (mock) intraperitoneally starting at day 61 after implantation for five days and/or MG18L (2 X 106 pfu) injected on day 63, and harvested on day 65. BT74 tumors (right) were treated similarly, except with olaparib starting on day 42, MG18L injected on day 44, and death on day 46. Lysates from tumor-bearing hemispheres from individual mice (1, 2, 3) were electrophoresed and probed with antibodies to PAR, poly(ADP-ribose) polymerase (PARP), cleaved PARP, γH2AX, Rad51, p-Chk1 (S345), Chk1, HSV-ICP8, and vinculin as loading control. All statistical tests were two-sided. Ola = olaparib; PARP = poly(ADP-ribose) polymerase.