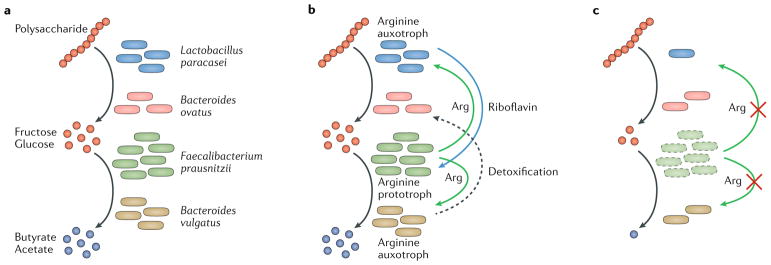
Fig. 1. Nutrient cycling in microbial communities.
a | Classic view of nutrient exchange involving successional interactions among members in a microbial community. Extracellular polysaccharides (for example, inulin, xylan or arabinogalactan) can be metabolized by human-associated bacteria, such as Bacteroides ovatus25 or Lactobacillus paracasei92, into different monosaccharides (for example, glucose or fructose). Breakdown products from saccharolytic bacteria in the gut can be converted to short-chain fatty acids (SCFAs; for example, acetate or butyrate) by primary fermenters, such as Faecalibacterium prausnitzii26 and Bacteroides vulgatus25, in a sequential manner. b | Expanding the classic view of electron donor exchange. Successional interactions among members in a microbial community (as described in part a) depend on the exchange of amino acids and vitamins. L. paracasei provides riboflavin (vitamin B2)93 for F. prausnitzii. Moreover, the arginine (Arg) prototroph F. prausnitzii supplies arginine for L. paracasei and B. vulgatus, which are both arginine auxotrophs. B. vulgatus additionally supports the growth of B. ovatus through the detoxification of inhibitory substances25. c | Perturbations (such as those that occur with antibiotic treatment or in a disease state) lead to changes in the dynamic equilibrium of microbial communities and alter the interactions between microorganisms. Reduced synthesis of arginine by F. prausnitzii limits its availability to arginine auxotrophs. In turn, growth of auxotrophs is decreased under nutrient-depleted conditions. The decrease in abundance of auxotrophs limits the availability of by-products, such as glucose and SCFAs, for other members in the community.