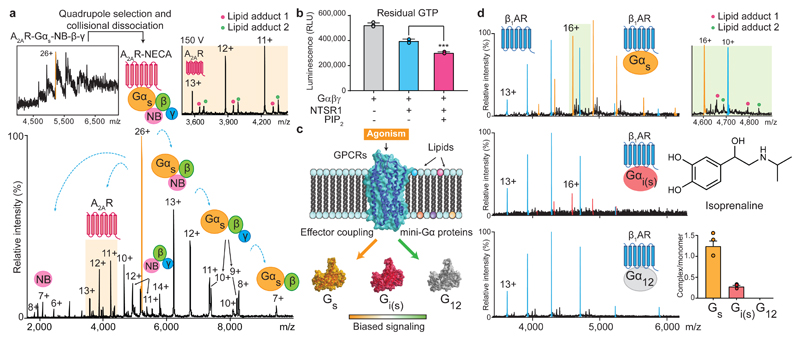
Figure 2. The selectivity of G-protein coupling and the presence of endogenous lipids on coupled receptors.
a, A representative mass spectrum of A2AR receptor coupled to a trimeric G-protein complex stabilised by a nanobody (Inset top left) from three independent experiments. Isolating and subjecting charge state 26+ (orange main figure) to collision-induced dissociation gives rise to subcomplexes and the liberated receptor with lipid adducts (highlighted orange). b, GTPase assays indicate an increase of GTP hydrolysis by active NTSR1coupled to trimeric Gαiβγ in the presence of PIP2 (*** denotes a statistically significant difference (p (0.0006) < 0.001) calculated with a t-test to compare the effect of PIP2 (one variable) on receptor-induced GTPase activation. Data points were overlaid and error bars represent standard deviations of the mean from three independent replicates. c, Schematic representation of the influence of lipids and agonists on the binding of mini-G proteins. d, Mass spectra of isoprenaline bound β1AR with three different mini-G subunits (mini-Gs, i(s),12). Enhanced coupling and lipid adducts are observed in the presence of Gs. (highlighted green top right). Error bars denote standard deviations of the mean from three independent replicates and each data point was overlaid.