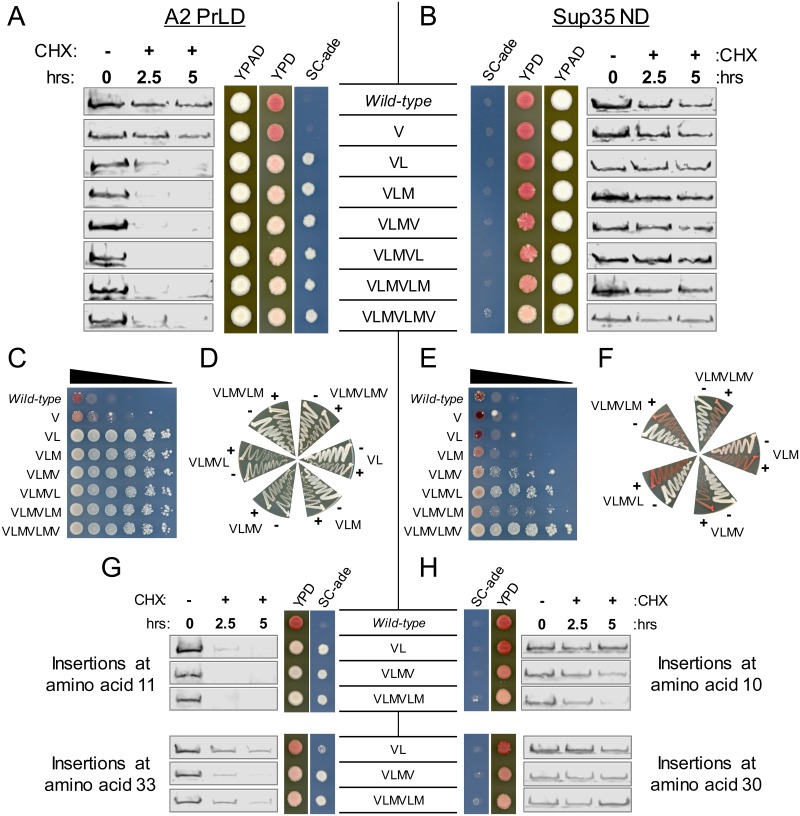
Fig 7. Degradation of the A2 PrLD and prion aggregation of Sup35 occur at similar hydrophobic content thresholds.
(A) Two or more hydrophobic residues inserted into the A2 PrLD resulted in a robust ADE+ phenotype and accelerated degradation of the A2 PrLD. (B) Insertion of three or more hydrophobic residues into Sup35 led to a progressive increase in the frequency of white sectors on YPD without affecting Sup35 turnover. (C) To quantify the frequency of ADE+ colony formation, serial dilutions of cells expressing each A2-Sup35 fusion were plated onto SC-ade. Degradation of the A2 PrLD upon insertion of two or more hydrophobic residues was correlated with a binary-like switch from ade- to ADE+. (D) ADE+ isolates from the A2 PrLD mutants were not curable by GuHCl. To test for curability of the ADE+ phenotype, individual ADE+ colonies were streaked on YPD (-) or YPD plus 4mM GuHCl (+), and then re-streaked onto YPD to test for loss of the ADE+ phenotype. (E) Insertion of three or more hydrophobic residues in the Sup35 ND leads to a progressive increase in ADE+ growth. (F) ADE+ isolates from the Sup35 mutants were curable by GuHCl, consistent with the ADE+ phenotype resulting from prion formation. (G) Insertion of hydrophobic amino acids at other positions in the A2 PrLD also promoted protein degradation. (H) Insertion of hydrophobic amino acids at other positions in the Sup35 nucleation domain had little or no effect on protein turnover.