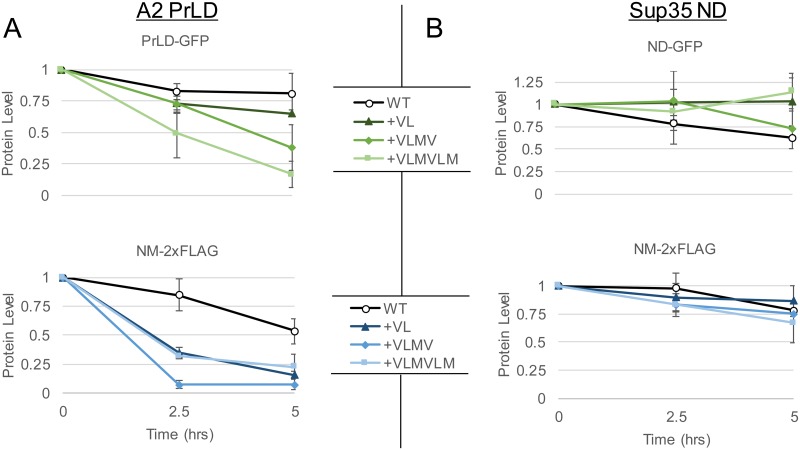
Fig 8. Degradation of the A2 PrLD and stability of the Sup35 ND upon insertion of hydrophobic residues do not depend on the Sup35 oligopeptide repeat domain (ORD), M-domain, or C-domain.
Progressively increasing hydrophobic content in the A2 PrLD (A) when fused to GFP alone (top) or with the remainder of the Sup35NM domains and tandem FLAG tags (bottom) enhances degradation rate of the A2 PrLD fusion proteins. By contrast, insertion of hydrophobic residues in the Sup35 ND (B) fused to GFP (top) or Sup35NM-2xFLAG (bottom) does not decrease stability of the Sup35 ND fusion proteins. Data represent means ± SDs (n = 3).