Abstract
Brincidofovir (CMX001) is an oral agent with activity against double-strand DNA viruses undergoing clinical trials in immunocompromised patients. We report a patient clinically diagnosed with brincidofovir-related gastrointestinal (GI) toxicity and his histologic findings. A 2-year-old boy with medulloblastoma undergoing autologous HCT developed adenovirus viremia 9 days post-transplant. After initial treatment with intravenous cidofovir he was started on oral brincidofovir as part of a clinical trial. He developed hematochezia, anorexia, and emesis 11 weeks later. Sigmoid colon biopsy showed marked crypt drop out, moderate epithelial apoptosis, and lamina propria edema. The pathologic diagnosis was drug-related injury versus infection. Brincidofovir toxicity was diagnosed clinically and the drug was discontinued. His GI symptoms improved in two weeks with supportive care and octreotide. Brincidofovir causes GI toxicity and histologically demonstrates epithelial apoptosis and crypt injury, similar to graft versus host disease and mycophenolate mofetil toxicity.
Keywords: Brincidofovir, CMX001, GVHD, graft versus host disease, hematopoietic cell transplant
Introduction
Brincidofovir (3-hexadecyloxy-1-propanol-cidofovir) is an orally bioavailable lipid conjugate of the nucleotide analog cidofovir with demonstrated activity against a broad spectrum of DNA viruses including cytomegalovirus (CMV), adenovirus (ADV), varicella zoster virus, herpes simplex virus, polyomaviruses (JC and BK virus), papillomaviruses, poxviruses, and mixed double-stranded DNA virus infections (1–21). The addition of a lipid moiety promotes more efficient absorption by enterocytes via both facilitated and passive diffusion, allowing brincidofovir to be orally dosed. Intracellularly, the prodrug is converted to cidofovir diphosphate by cleavage of its lipid moiety and phosphorylation by intracellular kinases (22–24). Unlike cidofovir, brincidofovir is not associated with nephrotoxicity or myelosuppression in Phase II clinical trials, potentially due to decreased plasma levels of the active drug (17).
Due to its broad antiviral activity and comparatively low risk of renal and marrow toxicity, brincidofovir shows promise in immunosuppressed patients. However, brincidofovir has been associated with significant gastrointestinal (GI) symptoms in hematopoietic cell transplantation (HCT) patients, including abdominal pain, diarrhea, nausea, and vomiting (1). There are numerous other etiologies associated with GI dysfunction in HCT patients including mucositis, infection (e.g., CMV, Clostridium difficile) and, primarily in the setting of allogeneic HCT, graft versus host disease (GVHD).
In certain clinical settings, it is not always possible to distinguish drug toxicity from other causes of GI symptoms (such as GVHD or infection) by clinical or laboratory findings alone. As the appropriate treatment varies dramatically depending on the etiology, biopsies are often performed to further elucidate the cause of GI symptoms. Herein we present a patient with an autologous HCT who was clinically diagnosed with brincidofovir-related GI toxicity. The clinical, endoscopic, and pathologic findings are reviewed; similarities between the histologic findings in this case and GVHD are highlighted and the potential implications of these findings for patient management are discussed.
Case Report
A 2-year-old boy diagnosed with medulloblastoma who underwent craniectomy for tumor resection and two cycles of chemotherapy. Eleven weeks after diagnosis he was conditioned with Carboplatin and Thiotepa and underwent autologous HCT. Within two weeks he developed septic shock, methicillin-sensitive Staphylococcus aureus and coagulase-negative staphylococcal bacteremia, C. difficile colitis, and ADV viremia and gastroenteritis. Repeated CMV PCR of his blood was negative.
His two episodes of bacteremia resolved less than one week after initiation of appropriate intravenous (IV) antibiotics. His C. difficile colitis was treated with Metronidazole and oral Vancomycin with repeat PCR testing negative 3 weeks later. His ADV gastroenteritis and viremia began 8 days after his transplant with a copy number of 171,000 DNA copies per mL blood and positive stool PCR (Table 1). He received two 5 mg/kg (81.75 mg) IV doses of cidofovir before being enrolled in a clinical trial for brincidofovir at 2 mg/kg (34 mg) orally twice weekly. ADV was undetectable in the blood and stool 5 and 18 days after initiation of brincidofovir therapy, respectively. He was maintained on brincidofovir for ADV prophylaxis.
Table 1.
Infectious etiology testing and therapy
| Days post-first HCT | ADV PCR blood (DNA copies/mL) | ADV PCR stool | Blood culture | Stool culture | Urine culture | CMV PCR blood | C. difficile PCR stool | Cidofovir | Brincidofovir |
|---|---|---|---|---|---|---|---|---|---|
| +0 | − | − | |||||||
| +4 | − | ||||||||
| +5 | + | ||||||||
| +6 | − | ||||||||
| +7 | − | − | |||||||
| +8 | + | − | − | ||||||
| +9 | + (171,000) | − | − | ||||||
| +10 | 1st dose | ||||||||
| +14 | + (<190) | − | − | ||||||
| +16 | + (392) | + | |||||||
| +17 | 2nd dose | ||||||||
| +21 | + (<190) | − | − | ||||||
| +24 | CNS | − | |||||||
| +26 | − | ||||||||
| +27 | − | ||||||||
| +28 | + (1100) | − | − | ||||||
| +30 | 1st dose | ||||||||
| +31 | − | ||||||||
| +35 | − | − | − | 2nd dose | |||||
| +38 | 3rd dose | ||||||||
| +40 | − | ||||||||
| +42 | − | − | − | 4th dose | |||||
| +44 | − | ||||||||
| +45 | 5th dose | ||||||||
| +48 | − | − | + | ||||||
| +49 | − | − | 6th dose | ||||||
| +51 | − | ||||||||
| +52 | 7th dose | ||||||||
| +53 | − | ||||||||
| +55 | − | − | |||||||
| +56 | − | + | − | − | − | − | − | 8th dose | |
| +57 | − | ||||||||
| +59 | − | 9th dose | |||||||
| +60 | − | ||||||||
| +63 | − | − | − | 10th dose | |||||
| +65 | − | ||||||||
| +66 | 11th dose | ||||||||
| +67 | − | ||||||||
| +70 | − | − | − | 12th dose | |||||
| +73 | 13th dose | ||||||||
| +76 | − | ||||||||
| +77 | − | − | − | 14th dose | |||||
| +79 | − | ||||||||
| +80 | 15th dose | ||||||||
| +81 | Rothia mucilaginosa | ||||||||
| +82 | − | ||||||||
| +83 | − | − | |||||||
| +84 (1st endoscopy) | − | 16th dose | |||||||
| +86 | − | ||||||||
| +87 | − | ||||||||
| +91 | − | − | |||||||
| +92 | − | − | |||||||
| +96 | − | ||||||||
| +98 (2nd endoscopy) | − | − | − | ||||||
| +99 | − | ||||||||
| +102 | − | ||||||||
| +103 | − | ||||||||
| +104 | − | ||||||||
| +105 | − | − | |||||||
| +112 | − | − | − |
ADV, adenovirus; C. difficile, Clostridium difficile; CMV, cytomegalovirus; CNS, coagulase negative Staphylococcus; HCT, hematopoietic stem cell transplant; PCR, polymerase chain reaction
After marrow recovery, he underwent a planned second autologous HCT. The first PCRs for ADV in the blood and stool after this HCT were negative. Five days after transplant his stool tested positive for C. difficile for which he received Metronidazole and oral Vancomycin with a negative stool PCR one week following completion of therapy. Two weeks after his second HCT he developed recurrent episodes of hematochezia, poor oral intake, delayed gastric emptying, and emesis lasting one month. On the day of GI symptom onset his stool tested positive for ADV by PCR; however, blood ADV PCR results remained negative. Throughout this time, he had continued to take brincidofovir biweekly. CMV and BK virus PCR of the blood were negative and a stool culture was negative for Salmonella, Shigella, Campylobacter, and Escherichia coli O157:H7. He underwent upper and lower GI endoscopies at 11 weeks of brincidofovir therapy. Biopsies were taken of the esophagus, gastric antrum, and sigmoid colon. ADV PCR of the stool 2 days after his endoscopy was negative. The patient was not on chemotherapy at the time of endoscopy and biopsy.
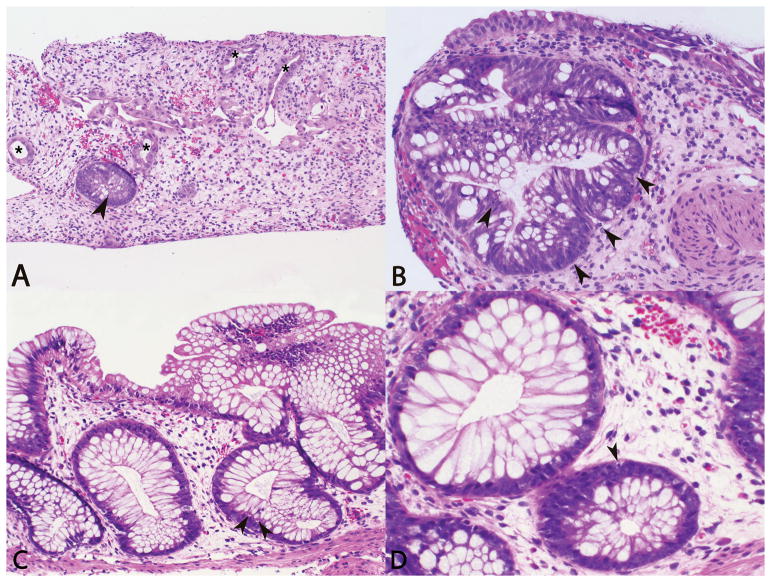
The esophageal and gastric biopsies were unremarkable. The gastric biopsy was negative for Helicobacter pylori, CMV, and ADV by immunohistochemistry (IHC). Conversely, the sigmoid colon biopsy showed marked crypt drop out, moderate epithelial apoptosis, reactive epithelial changes, and lamina propria edema (Image 1A,1B). The sigmoid colon biopsy was negative for CMV and ADV by IHC. No active inflammation, pseudomembrane formation, or increase in eosinophils was identified within this set of biopsies. The pathologic differential diagnoses for these findings in a HCT patient include drug related injury, infection, and GVHD. However, he had no cutaneous or hepatic signs or symptoms of GVHD, and GVHD is unlikely in cases of autologous HCT, so the former diagnostic differentials were favored. Following exclusion of an infectious etiology, brincidofovir toxicity was diagnosed clinically and the drug was discontinued.
Image 1.
Photomicrographs of sigmoid colon biopsy with marked crypt drop out, lamina propria edema, crypts with damage/reactive epithelial changes (asterisks), and moderate epithelial apoptosis (arrows) (A, H&E, 10X; B, H&E, 20X). Photomicrographs of follow up colon biopsy with mild lamina propria edema, reparative changes, and rare epithelial apoptosis (arrows) (C, H&E, 20X; D, H&E, 40X).
The patient’s GI function slowly improved with supportive care and octreotide. Follow up biopsies were performed 2 weeks after discontinuation of brincidofovir (Image 1C,1D). At the time of the biopsy the patient was feeling well without GI symptoms. ADV and CMV PCR on blood and C. difficile PCR on stool were negative. Blood culture and sigmoid colon viral culture were negative. The gastric biopsy showed chronic active gastritis. The gastric biopsy was negative for Helicobacter pylori and CMV by IHC. The sigmoid colon and rectum biopsies showed only focal crypt drop out, rare epithelial apoptosis, minimal lamina propria edema, and reparative changes with significant epithelial recovery compared to the previous biopsy; no significant acute inflammation was seen in the colonic biopsies. The sigmoid colon and rectum biopsies were negative for CMV and ADV by IHC. Eosinophils were not increased in any of the sites. The pathologic differential diagnosis was comparable to his previous biopsy with medication related toxicity being favored. Clinically these changes were favored to be residual/reparative changes from brincidofovir toxicity and no further treatment was initiated. The patient recovered, discontinued octreotide, and was discharged on oral medications and diet one month after discontinuation of brincidofovir.
Discussion
We report on a patient receiving brincidofovir post-autologous HCT with GI symptoms including hematochezia and vomiting. Histologic examination of a sigmoid colon biopsy showed marked crypt drop out, moderate epithelial apoptosis, and lamina propria edema. Of note, eosinophils were not increased. These histologic findings have overlapping features with several differential diagnoses of GI symptoms in the setting of an immunocompromised host, including infectious etiologies, GVHD, and medication toxicity.
All of the aforementioned etiologies can share common histologic features including epithelial apoptosis, reactive changes such as gland/crypt architectural distortion and mucin depletion, gland/crypt loss, and lymphoplasmacytic and neutrophilic inflammation. Viral etiologies may show more inflammation and viral cytopathic inclusions. Correlation with serology, PCR, and IHC are vital in improving sensitivity of detection of CMV infection. C. difficile colitis can show pseudomembrane formation with denuded epithelium, mucopurulent exudate, and karyorrhectic debris. Correlation with PCR is again vital to improving diagnostic sensitivity. Medication toxicity has been said to be associated with increased eosinophils; however, other studies have shown a direct correlation of tissue eosinophilia with GVHD severity (25, 26). GVHD is predominantly characterized by epithelial apoptosis but increased inflammation and mucosal injury is seen with increasing severity.
In this case, common infectious causes were ruled out by molecular testing and/or IHC studies including CMV (PCR on blood and IHC on biopsy), ADV (PCR on blood and stool and IHC on biopsy), C. difficile (PCR on stool), H. pylori (IHC on biopsy), BK virus (PCR on blood), and Salmonella, Shigella, Campylobacter, and Escherichia coli O157:H7 (stool culture). Additionally, the patient did not show signs or symptoms of skin or hepatic GVHD and he received an autologous HCT which carries a very low risk of GVHD. Resolution of the patient’s GI symptoms after discontinuation of brincidofovir, without another form of intervention, supports drug toxicity as the etiology of his symptoms and is the presumed cause of the observed histologic abnormalities. Additionally, a follow up biopsy of the stomach, sigmoid colon, and rectum were performed 2 weeks after the initial biopsy which showed marked improvement with only focal residual crypt drop out, rare epithelial apoptosis, and reparative changes. Infectious disease work up at this time was again negative for ADV (blood PCR and biopsy IHC), CMV (blood PCR and biopsy IHC), C. difficile (stool PCR), and H. pylori (biopsy IHC) as well as negative blood culture and sigmoid colon viral culture. Overall, these findings were clinically and histopathologically consistent with ongoing repair of drug-related injury, which can persist for months following discontinuation of GI-toxic agents (27, 28).
The exact mechanism of brincidofovir-related GI toxicity remains unknown. However, the current leading hypothesis is that toxicity results from the high levels of active cidofovir that accumulate in the intestinal epithelium when brincidofovir is orally dosed (17). Although IV cidofovir does not cause GI toxicity, it is toxic to the cells in which it accumulates (e.g., proximal tubular epithelial cells) via induction of apoptosis (29, 30).
The crypt drop out, moderate epithelial apoptosis, and lamina propria edema seen in this case suggest that brincidofovir toxicity should be considered within the differential for GI symptoms in immunosuppressed patients with GVHD-like changes on biopsy. There are examples in the literature of other GI-toxic agents used in transplant patients causing GVHD-like histological changes (e.g., mycophenolate mofetil) (31–34). However, to our knowledge this is the first report of GVHD-like histology in a patient with brincidofovir-related GI toxicity.
It is vital to the care of immunocompromised patients to distinguish between GI GVHD, infection, and drug-related GI toxicity. Although GI GVHD was unlikely given the patient received an autologous transplant, this case underscores the challenge that would occur if a similar presentation was in an allogeneic transplant. And in fact, we have faced this dilemma with a cohort of allogeneic HCT patients taking this medication at our institution. If GI symptoms in HCT transplant patients are incorrectly presumed to be due to GVHD, brincidofovir may be continued and administration of GVHD-directed therapies such as steroids may result in unnecessary toxicities and increased susceptibility to infection in these already vulnerable patients. Conversely, if the cause is GVHD and GI symptoms are incorrectly presumed to be due to brincidofovir toxicity, the patient may go without treatment for undiagnosed GVHD, leading to significant, potentially lethal complications. Furthermore, inappropriate discontinuation of brincidofovir in these cases will not relieve symptoms and may lead to increased susceptibility to infection by DNA viruses. Further studies are needed to try to elucidate more distinguishing features, but as long as there are overlapping histological features between brincidofovir toxicity and GVHD it is crucial to correlate the endoscopic and histological findings with the timeline of symptom development, clinical status, and the presence or absence of additional signs and symptoms of GVHD (e.g., skin findings). However, it is important to recognize that patients may present without signs or symptoms of GVHD involving other organs and/or the clinical timeline could fit with either GI GVHD or brincidofovir toxicity. In these instances, the recommendation is to discontinue brincidofovir, per the study protocol’s safety management plan, and, if deemed necessary, administer an alternative treatment for ADV.
In summary, our study demonstrates a novel case of brincidofovir-related toxicity with histologic findings including crypt drop out and epithelial apoptosis. In the future, we hope to build upon this case report by using a larger cohort of patients to systematically characterize the histological hallmarks of brincidofovir toxicity in the GI tract and to compare and contrast those findings with those of GVHD. Findings from these studies will help pathologists in accurately interpreting GI biopsies in patients taking brincidofovir and for clinicians to make informed decisions regarding treatment of GI symptoms in HCT patients.
Acknowledgments
Sources of Funding:
This work was supported by the National Institutes of Health [NRSA T32GM007171 to S.M., NCATS 5KL2TR001115 to A.S.]. For the remaining authors no sources of funding are declared.
Footnotes
Conflicts of Interest:
The authors declare no conflict of interest.
References
- 1.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–36. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 2.El-Haddad D, El Chaer F, Vanichanan J, et al. Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: A single-center experience. Antiviral Res. 2016;134:58–62. doi: 10.1016/j.antiviral.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18(5):731–8. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullane KM, Nuss C, Ridgeway J, et al. Brincidofovir treatment of acyclovir-resistant disseminated varicella zoster virus infection in an immunocompromised host. Transpl Infect Dis. 2016;18(5):785–90. doi: 10.1111/tid.12583. [DOI] [PubMed] [Google Scholar]
- 5.Quenelle DC, Kern ER. Treatment of Vaccinia and Cowpox Virus Infections in Mice with CMX001 and ST-246. Viruses. 2010;2(12):2681–95. doi: 10.3390/v2122681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrob Agents Chemother. 2011;55(10):4728–34. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price NB, Prichard MN. Progress in the development of new therapies for herpesvirus infections. Curr Opin Virol. 2011;1(6):548–54. doi: 10.1016/j.coviro.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang ZG, Cohen J, Marshall LJ, Major EO. Hexadecyloxypropyl-cidofovir (CMX001) suppresses JC virus replication in human fetal brain SVG cell cultures. Antimicrob Agents Chemother. 2010;54(11):4723–32. doi: 10.1128/AAC.00837-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Chemother. 2010;54(11):4714–22. doi: 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisman L, Habib S, McClure GB, Latiolais LS, Vanchiere JA. Treatment of BK virus-associated nephropathy with CMX001 after kidney transplantation in a young child. Pediatr Transplant. 2014;18(7):E227–31. doi: 10.1111/petr.12340. [DOI] [PubMed] [Google Scholar]
- 11.Papanicolaou GA, Lee YJ, Young JW, et al. Brincidofovir for polyomavirus-associated nephropathy after allogeneic hematopoietic stem cell transplantation. Am J Kidney Dis. 2015;65(5):780–4. doi: 10.1053/j.ajkd.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tylden GD, Hirsch HH, Rinaldo CH. Brincidofovir (CMX001) inhibits BK polyomavirus replication in primary human urothelial cells. Antimicrob Agents Chemother. 2015;59(6):3306–16. doi: 10.1128/AAC.00238-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calista D. Topical cidofovir for severe cutaneous human papillomavirus and molluscum contagiosum infections in patients with HIV/AIDS. A pilot study. J Eur Acad Dermatol Venereol. 2000;14(6):484–8. doi: 10.1046/j.1468-3083.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 14.Calista D. Resolution of recalcitrant human papillomavirus gingival infection with topical cidofovir. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(6):713–5. doi: 10.1067/moe.2000.110413. [DOI] [PubMed] [Google Scholar]
- 15.Rice AD, Adams MM, Wallace G, et al. Efficacy of CMX001 as a post exposure antiviral in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses. 2011;3(1):47–62. doi: 10.3390/v3010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the Treatment of Poxvirus Infections. Viruses. 2010;2(12):2740–62. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202(10):1492–9. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson VA, Smith SK, Foster S, et al. In vitro efficacy of brincidofovir against variola virus. Antimicrob Agents Chemother. 2014;58(9):5570–1. doi: 10.1128/AAC.02814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JI, Davila W, Ali MA, et al. Detection of molluscum contagiosum virus (MCV) DNA in the plasma of an immunocompromised patient and possible reduction of MCV DNA with CMX-001. J Infect Dis. 2012;205(5):794–7. doi: 10.1093/infdis/jir853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camargo JF, Morris MI, Abbo LM, et al. The use of brincidofovir for the treatment of mixed dsDNA viral infection. J Clin Virol. 2016;83:1–4. doi: 10.1016/j.jcv.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Dunning J, Kennedy SB, Antierens A, et al. Experimental Treatment of Ebola Virus Disease with Brincidofovir. PloS one. 2016;11(9):e0162199. doi: 10.1371/journal.pone.0162199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Painter W, Robertson A, Trost LC, Godkin S, Lampert B, Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56(5):2726–34. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82(2):A84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63(3):678–81. doi: 10.1124/mol.63.3.678. [DOI] [PubMed] [Google Scholar]
- 25.Star KV, Ho VT, Wang HH, Odze RD. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol. 2013;37(9):1319–28. doi: 10.1097/PAS.0b013e31829ab1ef. [DOI] [PubMed] [Google Scholar]
- 26.Daneshpouy M, Socie G, Lemann M, Rivet J, Gluckman E, Janin A. Activated eosinophils in upper gastrointestinal tract of patients with graft-versus-host disease. Blood. 2002;99(8):3033–40. doi: 10.1182/blood.v99.8.3033. [DOI] [PubMed] [Google Scholar]
- 27.Al-Absi AI, Cooke CR, Wall BM, Sylvestre P, Ismail MK, Mya M. Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc. 2010;42(9):3591–3. doi: 10.1016/j.transproceed.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 28.Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22(6):737–43. doi: 10.1038/modpathol.2009.44. [DOI] [PubMed] [Google Scholar]
- 29.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11(3):383–93. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz A, Justo P, Sanz A, et al. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther. 2005;10(1):185–90. [PubMed] [Google Scholar]
- 31.Papadimitriou JC, Cangro CB, Lustberg A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11(4):295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- 32.Papadimitriou JC, Drachenberg CB, Beskow CO, et al. Graft-versus-host disease-like features in mycophenolate mofetil-related colitis. Transplant Proc. 2001;33(3):2237–8. doi: 10.1016/s0041-1345(01)01951-0. [DOI] [PubMed] [Google Scholar]
- 33.Papadimitriou JC, Drachenberg CB, Wiland A, et al. Histological morphology of colitis associated with mycophenolate mofetil (MMF) in renal allogrft recipients: acute graft versus host disease (GVH)-like features. Transplantation. 1999;67(7):S256. [Google Scholar]
- 34.Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32(9):1367–72. doi: 10.1097/pas.0b013e31816bf3fe. [DOI] [PubMed] [Google Scholar]