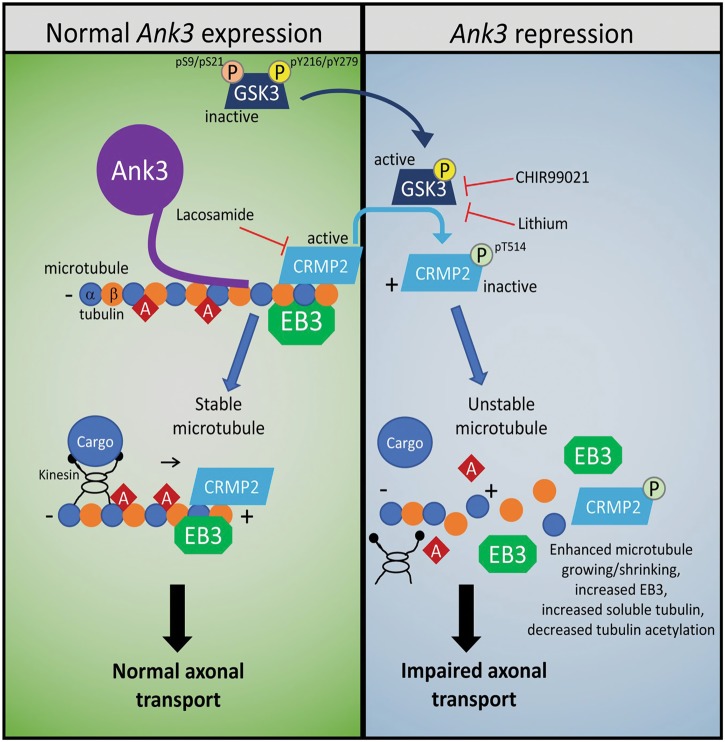
Fig. 6. Brain-specific Ank3 repression is associated with enhanced microtubule dynamics.
Left: Under normal Ank3 expression, microtubules are stabilized by binding of microtubule-associated proteins (MAPs), such as EB3 and CRMP2, to the microtubule plus end where α- and β-tubulin heterodimers polymerize to facilitate elongation of the microtubule. As microtubules elongate, EB3 and CRMP2 move along the growing plus end tip to stabilize newly generated microtubule segments. Acetylation (red diamond) accumulates on α-tubulin within the microtubule due to low microtubule turnover. Motor proteins, such as kinesin, mediate transport of cellular cargo along the microtubule towards the plus end, which is oriented towards the distal axon. Right: Repression of brain-specific Ank3 reduces phosphorylation of GSK3 (pS9/pS21) and increases GSK3 activity, leading to an increase in CRMP2 phosphorylation (pT514) and impaired CRMP2 binding and stabilization of microtubules. Microtubules become more susceptible to catastrophes, as demonstrated by increased EB3 expression, reduced EB3 comet length and duration, and increased ratio of soluble:polymerized tubulin. The increased susceptibility to catastrophes increases microtubule turnover and decreases acetylation of α-tubulin. Inhibition of GSK3 activity by lithium or CHIR99021 reduces CRMP2 phosphorylation (pT514), thereby allowing CRMP2 to bind and stabilize microtubules. Pharmacological inhibition of CRMP2 by lacosamide reduces CRMP2 binding and stabilization of microtubules, which increases the amount of free tubulin and decreases acetylation of α-tubulin. Enhanced microtubule dynamics induced by brain-specific Ank3 repression may have a range of effects (e.g. axonal transport of synaptic vesicles, microtubule interaction with motor proteins) that alter neuronal function