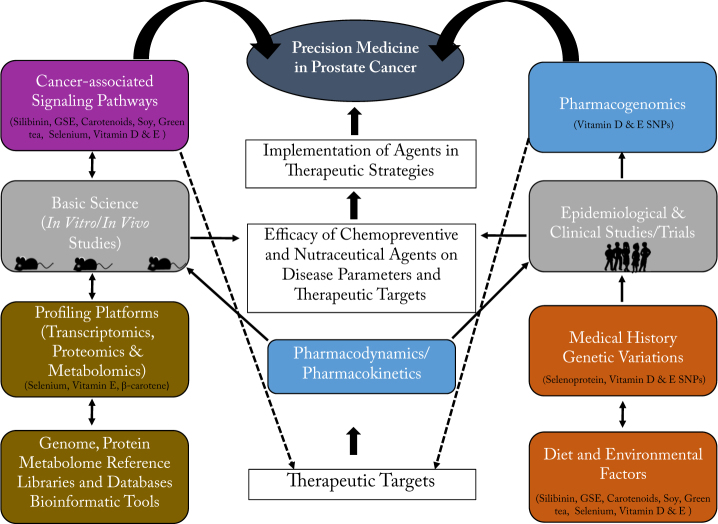
Fig. 1.
Nutraceutical efficacy in precision medicine. The schematic above depicts a workflow of experimental designs and assessment parameters required to establish the efficacy of nutraceutical agents. Therapeutic potential of agents must undergo vigorous pharmacodynamics and pharmacokinetics (blue) evaluation of their antioxidant and anti-cancer properties (e.g., anti-proliferation, anti-growth, anti-motility, anti-invasion) in cell models (gray). Targets that regulate altered tumor phenotypes are assessed via cell-focus assays (qRT-PCR, western blot, and immunofluorescence) and high-throughput platforms. Network analyses of targets are performed using omic profiling databases and libraries (brown) to determine gene ontology and identify therapeutic targets in cancer-associated canonical/non-canonical pathways (purple). Therapeutic targets and nutraceutical agents are evaluated in preclinical models and undergo the previous workflow. Next, nutraceutical agents are assessed in epidemiological studies and clinical trials (gray) which can evaluate hereditary, genetic, and environmental factors that range in degree from Phase I (≤30 patients, Pharmacodynamics/Pharmacokinetics parameters), Phase II (2–3 treatment groups including standard treatment + new agent, different doses, safety and toxicity assessments, and Pharmacogenomics in humans or animals), Phase III (comparison between new agent and standard treatments, Pharmacogenomics (blue)) (gray), Phase IV (Pharmacogenetic testing and side effects in different populations) to marketing and therapeutic application. After clinical trials have assessed the efficacy of nutraceuticals, these agents can be implemented in current precision medicine clinical therapeutic strategies for patients. The images of the mice and the group of people shown here are created by the authors.