Abstract
Background
Systemic inflammation is a cause of insulin dysregulation in many species, but the insulin and glucose dynamics in adult horses diagnosed with systemic inflammatory response syndrome (SIRS) are poorly documented.
Hypothesis/Objectives
In SIRS in horses, insulin and glucose dynamics will be altered and associated with survival.
Animals
Adult horses diagnosed with SIRS admitted to a referral hospital.
Methods
Prospective study enrolling horses diagnosed with SIRS in which serum insulin and glucose concentrations were measured. Horses were grouped by outcome (survival, hyperinsulinemia, and hyperglycemia) and compared with P < .05 considered significant.
Results
Fifty‐eight horses were included in the study and 36 (62%) survived. At admission, 21 horses (36%) were hyperinsulinemic and 44 horses (88%) were hyperglycemic, with survivors having significantly higher serum insulin and a significantly lower serum glucose concentration. Horses diagnosed with hyperinsulinemia at any time during hospitalization were 4 times more likely to survive whereas horses that were hyperglycemic at any time during hospitalization were 5 times less likely to survive. Serum glucose concentration and presence of hyperglycemia both were associated with severity of disease. Insulin/glucose ratio, reflecting insulin secretion, was significantly higher in survivors whereas glucose/insulin ratio, reflecting peripheral tissue insulin resistance, was significantly lower in nonsurvivors. Only in survivors was there a significant correlation between serum insulin and glucose concentrations.
Conclusions and Clinical Importance
Hyperinsulinemia and hyperglycemia are common features of SIRS in horses, but those presenting with relative hypoinsulinemia and corresponding hyperglycemia suggestive of endocrine pancreatic dysfunction have a worse prognosis.
Keywords: endocrinology, equine, glucose, inflammation, pancreas
Abbreviations
- EMS
equine metabolic syndrome
- OR
odds ratio
- LPS
lipopolysaccharide
- PPID
pituitary pars intermedia dysfunction
- ROC
receiver operating characteristic
- SIRS
systemic inflammatory response syndrome
1. INTRODUCTION
Systemic inflammation is associated with peripheral tissue insulin resistance and hyperglycemia in many species.1, 2, 3, 4 Insulin regulates lipid and carbohydrate metabolism by increasing glucose uptake from the blood to insulin‐sensitive tissues such as skeletal muscle, adipose tissue and liver.5 High concentrations of circulating insulin promote the transformation of glucose into glycogen by glycogenesis (skeletal muscles and liver) or into triglycerides by lipogenesis (adipose tissue and liver) whereas low concentrations of circulating insulin increase hepatic glucose secretion by promoting gluconeogenesis and glycogenolysis.5, 6 Insulin resistance is the failure of such tissues to respond to endogenous or exogenous insulin, resulting in uncontrolled hyperglycemia.7, 8 At a cellular level, insulin resistance is thought to develop by several mechanisms: decreased availability of the insulin receptor as a result of cellular hypoxia and oxidative stress causing activation of inflammatory pathways and inhibition of post‐receptor insulin signaling pathways.9
In people, peripheral tissue insulin resistance and hyperglycemia are described commonly with sepsis or severe trauma and high blood glucose concentration has been associated with increased risk of death and poor outcome.7, 8, 10, 11, 12 In critically ill patients, tight regulation of blood glucose concentration between 80 and 110 mg/dL by intensive insulin treatment has been associated with improved survival.8 However, other studies have shown that intensive insulin treatment significantly increases the risk of hypoglycemia, also associated with poor survival, conferring no overall mortality benefit among critically ill patients.13, 14, 15 Finally, other studies found that less stringent regulation of blood glucose concentration (between 140 and 180 mg/dL) by insulin treatment resulted in a decreased incidence of hypoglycemia without increased mortality rate, suggesting that cautious insulin treatment may be warranted in critically ill patients.16, 17, 18
In equids, systemic inflammation also has been associated with insulin dysregulation: lipopolysaccharide (LPS) infusion has been shown to result in peripheral tissue insulin resistance, and acute gastrointestinal disease results in hyperglycemia, also suggesting peripheral tissue insulin resistance.3, 19, 20 Similar to what has been described in people, in both foals and adult horses suffering from systemic diseases, hyperglycemia and peripheral tissue insulin resistance have been associated with increased mortality.21, 22 Nevertheless, several studies in horses also have reported low serum insulin concentration and improved insulin sensitivity in the early stages of systemic inflammation.20, 23, 24 For example, in sick foals, relative hypoinsulinemia with appropriate blood glucose concentration response has been described.25 In addition, septic foals have been reported to have increased insulin sensitivity in the early stages of sepsis.23 Similar observations have been made in adult horses in which transiently improved insulin sensitivity was documented after LPS infusion.20 In that study, acute pancreatic inhibition of insulin production in response to LPS was observed, suggesting that LPS‐associated hyperglycemia could result from pancreatic dysfunction in addition to peripheral insulin resistance.20 Furthermore, some hyperglycemic horses with systemic inflammatory response syndrome (SIRS) respond to small doses of exogenous insulin, suggesting adequate peripheral insulin sensitivity. These apparently conflicting studies indicate that, in sick horses, insulin and glucose dynamics are complex, varying with disease stage and severity, and that more data are necessary.
Although insulin treatment is recommended in hyperglycemic critically ill human patients, only anecdotal reports of the use of insulin treatment are available in horses with SIRS.14, 21 Possible explanations for this lack of use of insulin treatment in equine medicine are the fact that horses are not reported to develop pancreatic exhaustion as other species do and that hyperinsulinemia has been shown to induce laminitis within 48 hours of insulin administration in healthy horses.26, 27, 28
Several studies have used models of systemic inflammation to induce peripheral tissue insulin resistance, but no study has investigated insulin and glucose dynamics in spontaneously sick adult horses presented to a referral hospital. Our main objective was to describe glucose and insulin dynamics in horses diagnosed with SIRS and evaluate their usefulness as predictors of survival.
2. MATERIALS AND METHODS
2.1. Data collection
Adult horses (>5 years of age) with SIRS presented to the J.T. Vaughan Large Animal Teaching Hospital over a period of 20 months were recruited and serum insulin and glucose concentrations measured at admission. The diagnosis of SIRS was based on the presence of ≥ 2 of the following criteria: hyperthermia (>101.5°F or 38.6°C) or hypothermia (<97°F or 36.1°C), tachycardia (>45 beats/min), tachypnea (>24 breaths/min), leukopenia (white cell count <6000/μL), leukocytosis (white cell count > 12 000/μL), or > 10% band neutrophils.29 Horses then were divided into 4 groups based on the number of positive SIRS criteria (group 2 to group 5). Horses with any 2 SIRS criteria were allocated to group 2; any 3 SIRS criteria in group 3, and so on. For example, a horse presenting with pyrexia and leukopenia would be in group 2, whereas a horse with tachycardia, tachypnea, pyrexia, and leukopenia would be in group 4. Horses with clinical signs of pituitary pars intermedia dysfunction (PPID) and equine metabolic syndrome (EMS) were excluded, but no specific testing was performed to exclude mild or subclinical cases.30, 31
Data collected included signalment, physical examination findings at presentation, routine blood test results at presentation (hematologic and biochemical data), diagnosis, in‐hospital treatments (including surgery), duration of hospitalization, outcome, and type of intestinal lesion (ischemic lesion or not) for horses that underwent surgery or necropsy. Serum insulin and glucose concentrations were measured from samples collected at admission (after an estimated fasting time of at least 3 hours) and on days 2, 4, and 6 in the morning before feeding. In the first days of hospitalization, many horses were either anorexic, or having feed withheld or receiving minimal feed, limiting possible diet‐induced changes in insulin and glucose dynamics. Blood was collected by either venipuncture or through an aseptically placed IV catheter and placed in a plain glass tube. Blood was allowed to clot for 45 minutes at room temperature and centrifuged. Serum then was isolated and frozen at −80°C until assayed. Equine serum insulin was measured using a radioimmunoassay previously validated in horses (intra‐ and inter‐assay variation: 5.2% and 6.4%, respectively) and blood glucose concentration was measured using a glucohexokinase colorimetric assay as previously described.32, 33 A diagnosis of hyperinsulinemia was made if serum insulin concentration was >20 µIU/mL and a diagnosis of severe hyperinsulinemia was made if serum insulin concentration was >50 µIU/mL.34, 35 A diagnosis of hyperglycemia was made if serum glucose concentration was > 124 mg/dL (upper limit of diagnostic laboratory reference range). All aspects of the study were approved by the Auburn University Institutional Laboratory Animal Care and Use Committee and the College of Veterinary Medicine Clinical Research Review Committee, with signed owner consent obtained for all procedures.
2.2. Data analysis
Horses were categorized based on survival, hyperinsulinemia, hyperglycemia, and number of SIRS criteria and compared with P < .05 considered statistically significant. Normality was assessed by a Shapiro‐Wilk normality test. Data following a normal distribution were reported as mean ± SD and compared using an unpaired t‐test, whereas data not following a normal distribution were reported as median (range) and compared using a Mann‐Whitney U‐test. A receiver operating characteristic (ROC) curve was plotted to analyze the prognostic value of a given variable (serum insulin concentration, serum glucose concentration, or ratios). When > 2 groups were compared, an ANOVA was used for normally distributed data and a Kruskal‐Wallis test was used for non‐normally distributed data with a Dunn's post hoc test when appropriate. Categorical data were reported as counts and percentage of horses in which the variable was documented and compared using either a Chi‐square test or a Fisher's exact test, depending on expected counts. Odds ratios (OR) and 95% confidence intervals (CI) were calculated when appropriate. Statistical analysis was performed using commercially available statistical software (Prism, GraphPad Software, Inc, La Jolla, CA).
3. RESULTS
3.1. Animal population
Fifty‐eight horses met the inclusion criteria. Horses ranged from 5 to 32 years of age with a median age of 11 years. Twenty‐two horses (38%) were female and 36 (62%) were male, including 34 geldings (94% of males) and 2 stallions (6% of males). Breeds included Quarter Horse and associated breeds (25 horses, 43%), Thoroughbred (7 horses, 12%), Warmblood (5 horses, 9%), Arab (5 horses, 9%), Pony (3 horses, 5%), draft (3 horses, 5%), and mixed and other breeds (10 horses, 17%) reflecting the hospital population.
3.2. Clinical data
The most common clinical signs reported were tachycardia (53 horses, 91%), tachypnea (51 horses, 88%), prolonged capillary refill time (17 horses, 29%), and pyrexia (7 horses, 13%). Dehydration was recorded in 43 horses (74%) with a median estimation of 7% (4–12). Nasogastric reflux was present in 14 horses (24%) with a median volume of 5 L (2–14). Rectal palpation was performed in 50 horses (86%), and in 44 horses (88%) abnormal findings were described. The most commonly reported abnormal findings were distention of the small intestine (19 horses, 43%), impaction of the large colon (14 horses, 32%) and large colon displacement (8 horses, 18%). A CBC was available in 56 horses (97%) and a serum biochemistry profile was available in 50 horses (86%). The most commonly reported abnormalities were hyperglycemia (44 horses, 88%), hyperlactatemia (17 horses, 63%), neutrophilia (31 horses, 55%), hyponatremia (25 horses, 50%), hypokalemia (25 horses, 50%), hyperfibrinogenemia (13 horses, 42%), hypoalbuminemia (12 horses, 39%), increased serum creatinine concentration (19 horses, 38%), and decreased serum bicarbonate concentration (12 horses, 24%).
The final diagnosis involved the gastrointestinal system in 53 horses (91%), the respiratory system in 3 horses (5%), and the reproductive system and neurologic system in 1 case each (2% each). Fifteen horses with a gastrointestinal disease (28%) had an explorative laparotomy performed and, based on surgery or necropsy reports, 15 (28%) had ischemic lesions.
As per inclusion criteria, all of the horses were diagnosed with SIRS, with 31 horses (53%) in group 2, 18 horses (31%) in group 3 and 9 horses (16%) in group 4. None of the horses presented were allocated to group 5. Thirty‐six horses (62%) were discharged alive. Among the 22 horses that did not survive, 14 (64%) were euthanized because of poor prognosis; the reason for euthanasia was not recorded in the remaining cases. A necropsy was performed in 16 nonsurvivors (73%).
3.3. Insulin
Hyperinsulinemia (>20 µIU/mL) was diagnosed, at admission, in 21 horses (36%) and was not associated with survival (P = .21), but serum insulin concentration at admission was significantly higher in survivors than in nonsurvivors (12.6 µIU/mL [0.76‐55.16] versus 6.69 µIU/mL [0.06–24.34], P = .04, Figure 1). Presence of hyperinsulinemia at any time during hospitalization (Day 0, 2, 4, or 6) was associated with survival (P = .02, Table 1) with hyperinsulinemic horses 4 times more likely to survive. Severe hyperinsulinemia (insulin > 50 µIU/mL) was diagnosed in 6 horses but was not associated with survival (P = .39). Serum insulin and hyperinsulinemia at any time during hospitalization were not associated with SIRS group (P = .90 and P = .56, respectively).
Figure 1.
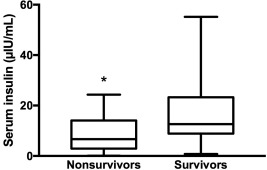
Serum insulin concentration (µIU/mL) at admission in nonsurvivors and survivors (*P < .05)
Table 1.
Categorical variables associated with survival
| Variables | OR | 95% CI | P‐value |
|---|---|---|---|
| Hyperglycemia at admission | 0.20 | 0.04–0.88 | .03 |
| Hyperinsulinemia during hospitalization | 4.03 | 1.16–12.4 | .02 |
The ROC curve showed that a serum insulin concentration >8.82 µIU/mL (the algorithm's suggested optimal cutoff) was a poor diagnostic test to predict survival with an area under the ROC of 0.67 yielding a sensitivity of 60.0% (36.1%‐80.9%) and specificity of 76.5% (58.8%‐89.3%).
3.4. Glucose
Hyperglycemia (>124 mg/dL) was diagnosed, at admission, in 44 horses (88%) and was associated with nonsurvival (P = .03, Table 1) with hyperglycemic horses 5 times less likely to survive. Serum glucose concentration at admission was significantly higher in nonsurvivors (165 mg/dL [90–387] versus survivors 137 mg/dL [45–329], P = .04, Figure 2). Serum glucose concentration and presence of hyperglycemia both were associated with SIRS group (P < .001, Figure 3, and P < .01, respectively).
Figure 2.
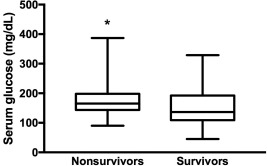
Serum glucose concentration (mg/dL) at admission in nonsurvivors and survivors (*P < .05)
Figure 3.
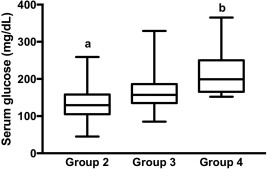
Serum glucose concentration (mg/dL) at admission in horses categorized by SIRS group 2, 3, and 4 (different letters indicate difference between groups, P < .05)
Using an optimal cutoff value of 150 mg/dL, the ROC curve showed that serum glucose concentration was a poor diagnostic test to predict survival with an area under the ROC curve of 0.66, yielding a sensitivity of 71.4% (47.8%‐88.7%) and a specificity of 63.9% (46.2%–79.2%).
3.5. Ratios and correlations
Insulin/glucose ratio, reflecting insulin secretion, was significantly higher in survivors than in nonsurvivors (0.1 [<0.01‐0.41] versus 0.033 [< 0.01‐0.22], P < .01, Figure 4). The ROC curve showed that an insulin/glucose ratio > 0.06 was a fair diagnostic test to predict survival with an area under the ROC of 0.75, yielding a sensitivity of 76.2% (52.8%‐91.8%) and specificity of 74.3% (56.7%‐87.5%).
Figure 4.
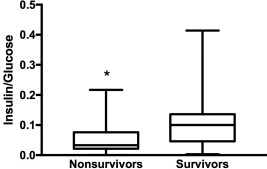
Insulin/glucose ratio, reflecting insulin secretion, in nonsurvivors, and survivors (*P < .05)
Glucose/insulin ratio, reflecting insulin sensitivity, was significantly lower in survivors (10 [2–62.4] versus nonsurvivors 28.5 [1.8–709.1], P < .01, Figure 5). A glucose/insulin ratio < 10, indicative of peripheral tissue insulin resistance, was found in 22 horses (38%) but was not associated with a final outcome of survival (P = .06).36 A glucose/insulin ratio < 4.5, indicative of severe peripheral tissue insulin resistance, was found in 8 horses (14%) but was not associated with survival (P = .11).36 Using an optimal cutoff value of 12.5, the ROC curve showed that the glucose/insulin ratio was a fair diagnostic test to predict survival with an area under the ROC curve of 0.73, yielding a sensitivity of 71.4% (47.8%‐88.7%) and specificity of 68.6% (50.7%‐83.2%).
Figure 5.
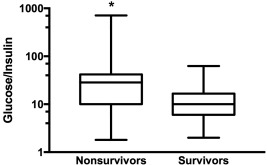
Glucose/insulin ratio, reflecting insulin sensitivity, in nonsurvivors, and survivors (*P < .05)
Overall, no correlation was found between serum insulin concentration and glucose concentration at admission (P = .13) but in survivors, a significant correlation was found between serum insulin and glucose concentrations (P = .002, R 2 = .25 Figure 6).
Figure 6.
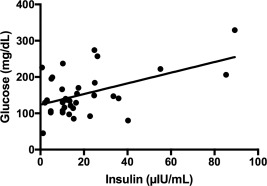
Correlation between serum insulin and glucose concentrations in survivors (P = .002, R 2 = .2468)
4. DISCUSSION
Our main finding was the association between hyperinsulinemia and favorable outcome and the association between hyperglycemia and poor outcome, suggesting that an appropriate pancreatic response to SIRS‐associated hyperglycemia is associated with survival.
Chronic or intermittent hyperinsulinemia is a component of insulin dysregulation and EMS, and usually is associated with poor prognosis because hyperinsulinemia results in laminitis.26, 31, 34, 37 The association between chronic or intermittent hyperinsulinemia and obesity is a well‐recognized phenomenon in equine endocrinology but limited data are available regarding the association between acute SIRS‐induced hyperinsulinemia and survival.38 Experimental infusion of LPS resulted in increases in serum insulin concentration, suggesting that hyperinsulinemia would develop in acutely sick horses.3, 20, 39 However, in 1 of these studies, insulin secretion was biphasic after LPS infusion.20 Although few horses were included in that study, LPS initially induced a mild decrease in serum insulin concentration before causing a more pronounced increase. This finding suggests inhibition of pancreatic β cell function in the early phases of endotoxemia. In foals, as well as in other species suffering from severe systemic inflammation, a state of relative hypoinsulinemia has been described.23, 40, 41 In those cases, relative hypoinsulinemia was defined as a failure of insulin secretion in the face of SIRS‐induced hyperglycemia, suggesting a transient diabetes mellitus, which is rarely reported in horses. Type‐1 diabetes mellitus, more commonly diagnosed in humans or in dogs, is observed as a consequence of immune‐mediated damage to the endocrine pancreas whereas type‐2 diabetes mellitus develops after decreased pancreatic β cell function after sustained hyperinsulinemia.42, 43 In 1 foal with severe SIRS, a transient type‐1 diabetes mellitus has been described suggesting that severe SIRS could lead to pancreatic dysfunction.44 In agreement with those reports, our data indicate that severe hyperglycemia, in the face of an inadequate insulin response, is associated with non‐survival and reflects a possible state of relative hypoinsulinemia or inappropriate lack of insulin secretion. In addition, although serum insulin concentration was significantly higher in survivors, it was rarely above reference range (20 µIU/mL) suggesting that, rather than excessive insulin secretion in survivors, decreased insulin secretion was observed in nonsurvivors. Taken together, these data suggest that, in horses, SIRS‐associated hyperglycemia is caused by peripheral tissue insulin resistance and by pancreatic dysfunction resulting in decreased insulin secretion.
In our study, hyperglycemia was associated with nonsurvival. Hyperglycemia previously has been associated with poor outcome in horses suffering from gastrointestinal diseases.21 In human medicine, large‐scale studies indicated that, in acutely ill patients in critical care units, prolonged hyperglycemia was associated with cardiovascular dysfunction, respiratory failure, increased odds of infection and worse neurological status.12, 45, 46, 47 In those studies, the explanations for hyperglycemia in critically ill patients included stress‐associated cortisol secretion, peripheral insulin resistance, hyperinsulinemia, and increased gluconeogenesis.14, 21 In our study, several variables such as serum insulin concentration and glucose/insulin ratio suggest that horses with SIRS also suffered from insulin dysregulation, at least partly caused by peripheral tissue insulin resistance. In our study, the larger the number of SIRS criteria, and presumably the sicker the animal, the higher the serum glucose concentration, suggesting that hyperglycemia could be used to estimate severity of SIRS in equine patients. However, given our small sample size, no actual validation of the grouping system could be performed and cautious interpretation is warranted. Nevertheless, in our sample, nonsurvivors had a significantly larger number of SIRS criteria than did survivors (P < .05). In critically ill people, serum glucose concentration is used as a prognostic indicator, and associations between specific outcomes and blood glucose concentration thresholds have been made.14 However, if serum glucose concentration is used to estimate the severity of disease, it should not be used to predict survival in horses with SIRS based on its mediocre sensitivity and specificity in our study.
In our study, the insulin/glucose ratio was lower in nonsurvivors. This ratio is used to estimate pancreatic insulin secretion in response to a glycemic challenge.34 Although the use of proxies, such as this ratio, has not been fully validated in horses, this difference between survivors and nonsurvivors confirms that the degree of pancreatic dysfunction in nonsurvivors was worse than in survivors. In our study, no dynamic testing was performed to assess pancreatic function, but our data indicate that horses with SIRS may experience a transient dysfunction of the endocrine pancreas. In horses, the main driver for insulin secretion is blood glucose concentration suggesting that in non‐survivors decreased insulin responsiveness and decreased insulin sensitivity could be responsible for the observed hyperglycemia.33 Therefore, although consistent with adequate insulin regulation, low serum insulin concentration in nonsurvivors would indicate relative hypoinsulinemia and transient endocrine pancreas dysfunction. However, although consistent with insulin dysregulation, the presence of hyperinsulinemia in survivors indicates an appropriate pancreatic response. The transient state of endocrine pancreas dysfunction observed in nonsurvivors also could explain the lack of association between serum insulin and glucose concentrations in that group. In our study, a significant correlation between serum insulin and glucose concentrations only was observed in survivors suggesting that, only in survivors, SIRS‐associated hyperglycemia results in an appropriate pancreatic response causing rebound hyperinsulinemia and limiting the deleterious effects of sustained hyperglycemia. If the state of relative hypoinsulinemia was prolonged because of a sustained inflammatory or infectious process, the prognosis would be worse. Therefore, in the context of SIRS, detection of hyperinsulinemia might indicate a better prognosis because it would imply an adequate pancreatic response to SIRS‐associated hyperglycemia.
In our study, none of the horses developed laminitis and no conclusion could be drawn on the association between hyperinsulinemia and laminitis in the presence of SIRS. Both hyperglycemia and sepsis have been associated with laminitis, but sepsis‐associated laminitis would be more likely a manifestation of multiorgan dysfunction syndrome rather than a consequence of acute hyperinsulinemia. Although similarities between insulin‐associated and sepsis‐associated laminitis have been documented at a molecular level, the different timings of the processes suggest that, if laminitis had been observed in our patients, the severity of the disease process would have had a larger effect on laminitis induction than the transient hyperinsulinemia observed in our study.48, 49, 50
A limitation of our study was the absence of dynamic testing to more fully assess the endocrine status of each horse during the episode of SIRS. Although EMS and PPID are well‐described, dynamic tests such as the oral glucose test, the 2‐step insulin response test and the thyrotropin‐releasing hormone stimulation test would have been required before or after the period of illness to completely eliminate subclinical EMS or PPID.51, 52, 53 Because our study involved spontaneous disease in client‐owned animals in which survivors were discharged after recovery, it was impossible to perform dynamic testing before or after the onset of SIRS. Nevertheless, in nonsurvivors in which necropsy was performed, no evidence of pituitary gland enlargement was found, suggesting that only a limited number of horses with PPID could have been recruited in our sample. Further work to validate the effects of dynamic tests of endocrine function in sick horses should be explored in future studies.
Our study is, to our knowledge, the first to provide evidence that horses with naturally occurring SIRS undergo transient pancreatic dysfunction and that persistence of that state, reflected by hyperglycemia and relative hypoinsulinemia, is associated with poor survival. Our study also showed that clinicopathologic evidence of insulin dysregulation, reflected by hyperinsulinemia and peripheral tissue insulin resistance, may not be associated with poor prognosis in the context of SIRS. In such cases, hyperinsulinemia might indicate a better prognosis, because it implies an intact endocrine pancreas capable of responding to SIRS‐associated hyperglycemia. Transient mild insulin dysregulation might therefore be an adaptive physiological state that promotes survival, whereas severe insulin dysregulation in the form of inappropriately low insulin concentrations in the face of hyperglycemia appears to be associated with a poor prognosis. As recommended in human medicine, cautious insulin treatment might be beneficial for horses with SIRS presenting with severe unregulated hyperglycemia.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All aspects of the study were approved by the Auburn University Institutional Laboratory Animal Care and Use Committee and the College of Veterinary Medicine Clinical Research Review Committee, with signed owner's consent obtained for all procedures.
ACKNOWLEDGMENTS
Taylor Towns, Heather Weaver, Bradley Johnson for assistance with sample collection, and Qiao Zhong for performing insulin assays. Many thanks to the family of Hall W. Thompson for supporting this research. Work was done at John Thomas Vaughan Large Animal Teaching Hospital, College of Veterinary Medicine, Auburn University, USA. Funding from Hoof Development and Rehabilitation Gift Fund. Presented in part as a research poster abstract at the European College of Equine Internal Medicine Congress in Budapest, Hungary, November 2017.
Bertin FR, Ruffin‐Taylor D, Stewart AJ. Insulin dysregulation in horses with systemic inflammatory response syndrome. J Vet Intern Med. 2018;32:1420–1427. 10.1111/jvim.15138
Funding information Hoof Development and Rehabilitation Gift Fund
REFERENCES
- 1. Torre DM, deLaforcade AM, Chan DL. Incidence and clinical relevance of hyperglycemia in critically ill dogs. J Vet Intern Med. 2007;21:971–975. [DOI] [PubMed] [Google Scholar]
- 2. Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126:879–887. [DOI] [PubMed] [Google Scholar]
- 3. Toth F, Frank N, Elliott SB, Geor RJ, Boston RC. Effects of an intravenous endotoxin challenge on glucose and insulin dynamics in horses. Am J Vet Res. 2008;69:82–88. [DOI] [PubMed] [Google Scholar]
- 4. Gross JJ, Wellnitz O, Bruckmaier RM. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows. J Anim Sci. 2015;93:3395–3401. [DOI] [PubMed] [Google Scholar]
- 5. Levine R, Goldstein MS, Huddlestun B, Klein SP. Action of insulin on the 'permeability' of cells to free hexoses, as studied by its effect on the distribution of galactose. Am J Physiol. 1950;163:70–76. [DOI] [PubMed] [Google Scholar]
- 6. Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85:69–79. [DOI] [PubMed] [Google Scholar]
- 7. Shangraw RE, Jahoor F, Miyoshi H, et al. Differentiation between septic and postburn insulin resistance. Metabolism. 1989;38:983–989. [DOI] [PubMed] [Google Scholar]
- 8. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 9. Stafeev IS, Vorotnikov AV, Ratner EI, Menshikov MY, Parfyonova YV. Latent inflammation and insulin resistance in adipose tissue. Int J Endocrinol. 2017;2017:5076732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. [DOI] [PubMed] [Google Scholar]
- 11. Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–3778. [DOI] [PubMed] [Google Scholar]
- 12. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. [DOI] [PubMed] [Google Scholar]
- 13. Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. [DOI] [PubMed] [Google Scholar]
- 14. Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132:268–278. [DOI] [PubMed] [Google Scholar]
- 15. Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data. CMAJ. 2009;180:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Preiser JC, Devos P, Ruiz‐Santana S, et al. A prospective randomised multi‐centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–1748. [DOI] [PubMed] [Google Scholar]
- 17. Marik PE. Glycemic control in critically ill patients: what to do post NICE‐SUGAR? World J Gastrointest Surg. 2009;1:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van den Berghe G, Schetz M, Vlasselaers D, et al. Clinical review: Intensive insulin therapy in critically ill patients: NICE‐SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94:3163–3170. [DOI] [PubMed] [Google Scholar]
- 19. Hollis AR, Boston RC, Corley KT. Blood glucose in horses with acute abdominal disease. J Vet Intern Med. 2007;21:1099–1103. [DOI] [PubMed] [Google Scholar]
- 20. Tadros EM, Frank N, De Witte FG, Boston RC. Effects of intravenous lipopolysaccharide infusion on glucose and insulin dynamics in horses with equine metabolic syndrome. Am J Vet Res. 2013;74:1020–1029. [DOI] [PubMed] [Google Scholar]
- 21. Hassel DM, Hill AE, Rorabeck RA. Association between hyperglycemia and survival in 228 horses with acute gastrointestinal disease. J Vet Intern Med. 2009;23:1261–1265. [DOI] [PubMed] [Google Scholar]
- 22. Hollis AR, Furr MO, Magdesian KG, et al. Blood glucose concentrations in critically ill neonatal foals. J Vet Intern Med. 2008;22:1223–1227. [DOI] [PubMed] [Google Scholar]
- 23. Barsnick RJ, Hurcombe SD, Smith PA, et al. Insulin, glucagon, and leptin in critically ill foals. J Vet Intern Med. 2011;25:123–131. [DOI] [PubMed] [Google Scholar]
- 24. Vick MM, Murphy BA, Sessions DR, et al. Effects of systemic inflammation on insulin sensitivity in horses and inflammatory cytokine expression in adipose tissue. Am J Vet Res. 2008;69:130–139. [DOI] [PubMed] [Google Scholar]
- 25. Armengou L, Jose‐Cunilleras E, Rios J, Cesarini C, Viu J, Monreal L. Metabolic and endocrine profiles in sick neonatal foals are related to survival. J Vet Intern Med. 2013;27:567–575. [DOI] [PubMed] [Google Scholar]
- 26. de Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42:129–135. [DOI] [PubMed] [Google Scholar]
- 27. Reeves HJ, Lees R, McGowan CM. Measurement of basal serum insulin concentration in the diagnosis of Cushing's disease in ponies. Vet Rec. 2001;149:449–452. [DOI] [PubMed] [Google Scholar]
- 28. McGowan CM, Frost R, Pfeiffer DU, Neiger R. Serum insulin concentrations in horses with equine Cushing's syndrome: response to a cortisol inhibitor and prognostic value. Equine Vet J. 2004;36:295–298. [DOI] [PubMed] [Google Scholar]
- 29. Arroyo MG, Slovis NM, Moore GE, Taylor SD. Factors associated with survival in 97 horses with septic pleuropneumonia. J Vet Intern Med. 2017;31:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McFarlane D. Equine pituitary pars intermedia dysfunction. Vet Clin North Am Equine Pract. 2011;27:93–113. [DOI] [PubMed] [Google Scholar]
- 31. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J Vet Intern Med. 2010;24:467–475. [DOI] [PubMed] [Google Scholar]
- 32. Reimers TJ, Cowan RG, McCann JP, Ross MW. Validation of a rapid solid‐phase radioimmunoassay for canine, bovine, and equine insulin. Am J Vet Res. 1982;43:1274–1278. [PubMed] [Google Scholar]
- 33. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310:E61–E72. [DOI] [PubMed] [Google Scholar]
- 34. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49:570–576. [DOI] [PubMed] [Google Scholar]
- 35. Frank N, Bailey S, Durham A, Kritchevsky J, Menzies‐Gow N, Tadros L. The Equine Endocrinology Group 2015 Recommendations for the Diagnosis and Treatment of EMS; 2015. Available at https://sites.tufts.edu/equineendogroup/files/2016/11/2016-11-2-EMS-EEG-Final.pdf. Accessed April 4, 2018.
- 36. Frank N. Equine metabolic syndrome. Vet Clin North Am Equine Pract. 2011;27:73–92. [DOI] [PubMed] [Google Scholar]
- 37. Menzies‐Gow NJ, Harris PA, Elliott J. Prospective cohort study evaluating risk factors for the development of pasture‐associated laminitis in the United Kingdom. Equine Vet J. 2017;49:300–306. [DOI] [PubMed] [Google Scholar]
- 38. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103–112. [DOI] [PubMed] [Google Scholar]
- 39. Toribio RE, Kohn CW, Hardy J, Rosol TJ. Alterations in serum parathyroid hormone and electrolyte concentrations and urinary excretion of electrolytes in horses with induced endotoxemia. J Vet Intern Med. 2005;19:223–231. [DOI] [PubMed] [Google Scholar]
- 40. van Waardenburg DA, Jansen TC, Vos GD, Buurman WA. Hyperglycemia in children with meningococcal sepsis and septic shock: the relation between plasma levels of insulin and inflammatory mediators. J Clin Endocrinol Metab. 2006;91:3916–3921. [DOI] [PubMed] [Google Scholar]
- 41. Leininger MT, Portocarrero CP, Schinckel AP, et al. Physiological response to acute endotoxemia in swine: effect of genotype on energy metabolites and leptin. Domest Anim Endocrinol. 2000;18:71–82. [DOI] [PubMed] [Google Scholar]
- 42. O'Kell AL, Wasserfall C, Catchpole B, et al. Comparative pathogenesis of autoimmune diabetes in humans, NOD mice, and canines: has a valuable animal model of Type 1 diabetes been overlooked? Diabetes. 2017;66:1443–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chandler M, Cunningham S, Lund EM, et al. Obesity and associated comorbidities in people and companion animals: A One Health perspective. J Comp Pathol. 2017;156:296–309. [DOI] [PubMed] [Google Scholar]
- 44. Navas de Solis C, Foreman JH. Transient diabetes mellitus in a neonatal Thoroughbred foal. J Vet Emerg Crit Care (San Antonio). 2010;20:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suematsu Y, Sato H, Ohtsuka T, Kotsuka Y, Araki S, Takamoto S. Predictive risk factors for delayed extubation in patients undergoing coronary artery bypass grafting. Heart Vessels. 2000;15:214–220. [DOI] [PubMed] [Google Scholar]
- 46. Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;59:80–83. [DOI] [PubMed] [Google Scholar]
- 47. Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. [DOI] [PubMed] [Google Scholar]
- 48. Bertin FR, Reising A, Slovis NM, Constable PD, Taylor SD. Clinical and clinicopathological factors associated with survival in 44 horses with equine neorickettsiosis (Potomac horse Fever). J Vet Intern Med. 2013;27:1528–1534. [DOI] [PubMed] [Google Scholar]
- 49. Stewart MC, Hodgson JL, Kim H, Hutchins DR, Hodgson DR. Acute febrile diarrhoea in horses: 86 cases (1986–1991). Aust Vet J. 1995;72:41–44. [DOI] [PubMed] [Google Scholar]
- 50. de Laat MA, van Eps AW, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: comparative histopathology 48 hours after experimental induction with insulin or alimentary oligofructose in Standardbred horses. J Comp Pathol. 2011;145:399–409. [DOI] [PubMed] [Google Scholar]
- 51. Beech J, Boston R, Lindborg S. Comparison of cortisol and ACTH responses after administration of thyrotropin releasing hormone in normal horses and those with pituitary pars intermedia dysfunction. J Vet Intern Med. 2011;25:1431–1438. [DOI] [PubMed] [Google Scholar]
- 52. Bertin FR, Sojka‐Kritchevsky JE. Comparison of a 2‐step insulin‐response test to conventional insulin‐sensitivity testing in horses. Domest Anim Endocrinol. 2013;44:19–25. [DOI] [PubMed] [Google Scholar]
- 53. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2017;49(2):238–243. [DOI] [PubMed] [Google Scholar]


