Abstract
Background
Hypomagnesemia is associated with increased mortality and renal function decline in humans with chronic kidney disease (CKD). Magnesium is furthermore inversely associated with fibroblast growth factor 23 (FGF23), an important prognostic factor in CKD in cats. However, the prognostic significance of plasma magnesium in cats with CKD is unknown.
Objectives
To explore associations of plasma total magnesium concentration (tMg) with plasma FGF23 concentration, all‐cause mortality, and disease progression in cats with azotemic CKD.
Animals
Records of 174 client‐owned cats with IRIS stage 2‐4 CKD.
Methods
Cohort study. Cats with azotemic CKD were identified from the records of two London‐based first opinion practices (1999–2013). Possible associations of baseline plasma tMg with FGF23 concentration and risks of death and progression were explored using, respectively, linear, Cox, and logistic regression.
Results
Plasma tMg (reference interval, 1.73–2.57 mg/dL) was inversely associated with plasma FGF23 when controlling for plasma creatinine and phosphate concentrations (partial correlation coefficient, −0.50; P < .001). Hypomagnesemia was observed in 12% (20/174) of cats, and independently associated with increased risk of death (adjusted hazard ratio, 2.74; 95% confidence interval [CI], 1.35–5.55; P = .005). The unadjusted associations of hypermagnesemia (prevalence, 6%; 11/174 cats) with survival (hazard ratio, 2.88; 95% CI, 1.54–5.38; P = .001), and hypomagnesemia with progressive CKD (odds ratio, 17.7; 95% CI, 2.04–154; P = .009) lost significance in multivariable analysis.
Conclusions and Clinical Importance
Hypomagnesemia was associated with higher plasma FGF23 concentrations and increased risk of death. Measurement of plasma tMg augments prognostic information in cats with CKD, but whether these observations are associations or causations warrants further investigation.
Keywords: feline, FGF23, phosphate, survival, renal
Abbreviations
- CI
confidence interval
- CKD
chronic kidney disease
- CKD‐MBD
chronic kidney disease‐mineral and bone disorder
- FGF23
fibroblast growth factor 23
- HR
hazard ratio
- IRIS
International Renal Interest Society
- OR
odds ratio
- PTH
parathyroid hormone
- RERI
relative excess risk due to interaction
- SBP
systolic blood pressure
- tMg
total magnesium concentration
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
- UTI
urinary tract infection.
1. INTRODUCTION
Hyperphosphatemia is a well‐documented sequela of chronic kidney disease (CKD) in cats,1, 2, 3, 4, 5 and associated with increased risks for death and progression of azotemia.6, 7, 8 Actions of the body to maintain normophosphatemia result in secondary renal hyperparathyroidism and fibroblast growth factor 23 (FGF23) excess.9 These hormonal derangements prevent overt hyperphosphatemia in the early stages of CKD,10 but contribute to bone pathology and soft tissue calcification.9, 11, 12 The systemic condition of disturbed mineral metabolism, bone disease, and extraskeletal calcification caused by CKD has been termed CKD‐mineral and bone disorder (CKD‐MBD).9, 11, 13 Dietary phosphate restriction is the mainstay of management of CKD‐MBD in cats, and has been shown to reduce plasma phosphate, FGF23, and PTH concentrations, and to improve survival.14, 15, 16, 17, 18
Recently, there is increased interest in the role of magnesium in human CKD‐MBD. Magnesium is an essential mineral for numerous intracellular processes,19 but also an inhibitor of vascular calcification20, 21, 22 and the release of profibrotic cytokines.23 Hypomagnesemia is a risk factor for death,24, 25, 26, 27, 28 and possibly for kidney function decline in human CKD patients.23, 24 Magnesium furthermore appears to modify the risks associated with hyperphosphatemia in humans with CKD, as high phosphate was only associated with higher risks of death27 and progression to end‐stage renal disease23 in patients with lower serum total magnesium concentrations (tMg). Interestingly, magnesium might be involved in FGF23 regulation, because an inverse association between these two variables was observed in human CKD patients on hemodialysis,29 and serum FGF23 concentrations were increased in rodents fed a magnesium deficient diet.30, 31 Plasma FGF23 itself is a strong predictor of survival and progression in cats with CKD.5
Little is known about the role of magnesium in feline CKD‐MBD. A study among cats found significantly increased plasma tMg in end‐stage CKD, whilst low plasma tMg was observed in up to 25% of cats with earlier stages of CKD.3 Neither the prognostic significance of magnesium status, nor the relationship between plasma tMg and FGF23 have been examined in cats with CKD. Therefore, we aimed to explore, first, the prevalence and risk factors for magnesium disorders in cats with azotemic CKD, second, the relationship of plasma tMg with FGF23 and other clinicopathological variables, and third, the prognostic significance of magnesium disorders for all‐cause mortality and renal function decline in a cohort of cats with azotemic CKD.
2. METHODS
2.1. Case selection
Cats were identified from the clinical records of two first opinion practices in central London (People's Dispensary for Sick Animals in Bow and Beaumont Sainsbury Animal Hospital in Camden). Client‐owned cats ≥9 years old visited these clinics for general health screening, and those diagnosed with azotemic CKD subsequently for management of their disease. Cats enrolled in our study were part of a larger observational cohort for which owner consent was obtained and approval of the Ethics and Welfare Committee of the Royal Veterinary College had been granted.
A group of 120 apparently healthy cats ≥9 years seen between September 2001 and September 2013 was selected to establish a reference interval for plasma tMg in older cats. Cats were considered apparently healthy if no significant abnormalities were detected in the clinical history, physical examination, or blood and urine examination, and if no medications had been prescribed. For inclusion, a stored heparinized plasma sample had to be available for measurement of tMg.
A cohort of cats diagnosed with azotemic CKD between August 1999 and July 2013 was selected in which to explore the clinical significance of baseline plasma tMg in feline CKD. Criteria for a diagnosis of azotemic CKD were plasma creatinine concentration ≥2 mg/dL in conjunction with a urine specific gravity (USG) <1.035, or plasma creatinine concentration ≥2 mg/dL on 2 consecutive occasions 2–4 weeks apart. To be enrolled, data on plasma FGF23 concentration and a stored residual heparinized plasma sample for measurement of tMg had to be available from the time of diagnosis of CKD. Cats with clinical signs of hyperthyroidism, plasma total thyroxine concentration >40 nmol/L, medical treatment for hyperthyroidism, diabetes mellitus, or treatment with corticosteroids were excluded from all analyses. Cats receiving amlodipine besylate for treatment of systemic hypertension were included.
2.2. Data collection
Data obtained at diagnosis of azotemic CKD were retrieved from electronic clinical records, and included age, breed, sex, body weight, body condition score (BCS; 9‐point system), muscle mass score (4‐point system), systolic blood pressure (SBP), PCV, routine plasma biochemical variables (total protein, albumin, globulin, creatinine, sodium, potassium, chloride, cholesterol, phosphate, and total calcium concentrations), ionized calcium concentration, venous blood gases and pH values, plasma calcidiol, calcitriol, FGF23, PTH, and total thyroxine concentrations, USG, urine culture result, and urine protein‐to‐creatinine ratio (UPC). The date of death and whether progression of azotemia occurred (defined below) were also documented. Anomalous or missing data from the electronic records were verified by consulting the physical patient records. Severity of CKD and phosphate status were classified according to International Renal Interest Society (IRIS) guidelines (International Renal Interest Society Guidelines: IRIS Staging of CKD. http://iris-kidney.com/guidelines/staging.html).
Plasma tMg was measured after enrollment of cats to the cohort so the selection process was blinded to the exposure of interest. It was measured in residual heparinized plasma, which had been stored at −80°C, by the laboratory that also had performed the routine biochemical analysis (Idexx laboratories, Wetherby, UK). Blood samples had been obtained via jugular venipuncture and urine samples via cystocentesis. Intact FGF23 and PTH were measured in EDTA plasma using validated4, 32 ELISA (FGF‐23 ELISA Kit, Kainos Laboratories, Tokyo, Japan) and immunoradiometric (Total intact PTH immunoradiometric assay–coated bead version, 3KG600, Scantibodies, Santee, California) assays, respectively. For measurement of FGF23, samples were diluted with the zero standard to achieve a reading on the standard curve. The PTH assay had a limit of detection of 5.2 pg/mL,32 and samples with a concentration below this value were assigned an arbitrary PTH concentration of 2.6 pg/mL. Urinalysis included in‐house measurement of USG by refractometry, dipstick analysis, and urine sediment microscopic examination. Urinary tract infections (UTI) were confirmed by culture (Royal Veterinary College Diagnostic Laboratory Services, Hatfield, UK). Urine biochemistry was performed by a commercial laboratory (Idexx laboratories), and UPC values of cats with a UTI were omitted. Systolic BP was assessed using the Doppler method (Parks Electronic Doppler Model 811B; Perimed UK, Bury St Edmunds, UK), and indirect ophthalmoscopy was performed in all cats where SBP >160 mm Hg was identified. Systemic hypertension was defined as SBP >170 mm Hg on at least 2 occasions 1–2 weeks apart, or a single SBP >160 mm Hg in association with ocular target organ damage.
2.3. Statistical analysis
Statistical analyses were performed using statistical software packages (IBM SPSS Statistics for Windows, Version 24, IBM Corp., Armonk, New York and GraphPad Prism 7, GraphPad Software, La Jolla, California). For all reported analyses, two‐sided tests of significance were carried out with a type I error rate <0.05 defining statistical significance. Continuous clinical data are presented as mean (SD) or as median [25th, 75th percentiles] as appropriate. The distribution of numerical variables was assessed for normality by Shapiro‐Wilk test and visual inspection of quantile‐quantile plots. Groups were compared using either independent samples t‐test (2 groups) or one‐way ANOVA with Bonferroni post hoc comparison (≥3 groups) for continuous variables with a normal distribution, or using Mann‐Whitney U tests (2 groups) or Kruskal‐Wallis test followed by Dunn's post hoc comparison (≥3 groups) for variables with a skewed distribution. Proportions were compared using Fisher's exact test.
Next to plasma tMg, the following variables were included in the regression analyses presented below: age, sex, body weight, BCS, plasma creatinine, phosphate, total calcium, cholesterol, sodium, chloride, potassium, albumin, globulin, FGF23, and PTH concentrations, PCV, USG, UPC, hypertension status, and presence of a bacterial UTI. Data on muscle mass score (n = 68), ionized calcium and venous blood gases (n = 47), and vitamin D‐metabolites (n = 17) were available in <50% of cats, therefore these variables were excluded from analysis.
2.4. Prevalence and factors associated with magnesium disorders
Cats with azotemic CKD were categorized in 3 groups based on the lower and upper limits of the reference interval for plasma tMg derived from apparently healthy cats ≥9 years old, which was calculated using the parametric method (ie, mean ± 2SD), and baseline characteristics among the 3 magnesium groups compared. Binary logistic regression was performed to explore risk factors for hypomagnesemia or hypermagnesemia, with normomagnesemic cats as controls. Plasma FGF23 and PTH were log‐transformed before analysis (logarithmus naturalis [ln]). Variables significantly associated with these disorders (P < .05) were entered into a multivariable binary logistic regression model. The final linear model was derived by manual backward elimination. Goodness of fit of the model was assessed with Hosmer–Lemeshow test. Results are reported as odds ratio (OR; 95% confidence interval [CI]).
2.5. Association of plasma total magnesium with plasma FGF23 and other clinicopathological variables
The Pearson correlation coefficient (r) was computed to evaluate the association between plasma tMg and log‐transformed FGF23 concentration. Partial correlation was performed to measure the strength of association between these two variables with the confounding effects of ln[creatinine] and ln[phosphate] removed, both known predictors of plasma FGF23.4
Univariable general linear models adjusted for IRIS stage were constructed to explore what variables were associated with plasma tMg as a continuous variable. Plasma creatinine, phosphate, FGF23, and PTH concentrations, and UPC were log‐transformed before analysis. IRIS stage, sex, and hypertension status were entered as categorical variables. Covariates associated with plasma tMg with P <0.10 were assessed for statistical interaction with IRIS stage and entered into a multivariable linear regression model including any significant interaction terms (P < .05). The final regression model was derived by backward elimination. The assumptions of normality and of linear relationship among variables were checked by visual inspection of histograms of the residuals and of scatter plots of the residuals against the fitted values. Results are reported as regression coefficient (β; 95% CI).
2.6. Association of plasma total magnesium with survival
To assess if plasma tMg was related to survival, all cats were included in a survival analysis for which the date of diagnosis of azotemic CKD was designated as baseline, death of all‐causes was the event of interest, and censoring occurred for cats that were lost to follow‐up or that were still alive on July 1, 2016. Cats lost to follow‐up were censored on the last date they were known to be alive. The Kaplan Meier curve of the normomagnesemic group was compared with those of the hypomagnesemic and hypermagnesemic cats using log‐rank test, and hazard ratios (HR) were calculated with univariable time‐invariant Cox proportional hazard analysis. Multivariable Cox regression was performed to adjust for possible confounding factors. Continuous variables were categorized if the assumption of proportional hazards, evaluated by inspection of Kaplan‐Meier curves and assessment of statistical interaction of each variable with time, were not met. Grouping was based on clinically relevant margins if possible (plasma tMg, hypertension status, BCS), or terciles (phosphate, FGF23, sodium, USG, weight). Plasma PTH was log‐transformed because of its strongly skewed distribution. No missing data imputation was performed. Variables associated with survival with P <0.10 were assessed for interaction with magnesium status, and subsequently entered multivariable analysis together with any statistically significant interaction term (P < .05). The final multivariable model was derived by manual backward elimination. The overall fit of the Cox model was checked by visual inspection of a Cox‐Snell residual plot. Results are reported as HR (95% CI).
Given the nonlinear relationship with mortality rates, plasma tMg was analyzed as a categorical variable divided on the lower and upper limits of the derived reference interval in the main analysis. To explore the effect of plasma tMg on survival on a continuous scale, a subanalysis was performed. Cats were divided by a median split of tMg, and instead of as a categorical variable, tMg entered in the fully adjusted Cox model as a continuous variable.
2.7. Association of plasma total magnesium with progression of CKD
Whether plasma tMg was associated with CKD progression was examined using binary logistic regression. Cats were categorized into 2 groups: a progressive CKD group that showed >25% increase in plasma creatinine concentration within the first 12 months of diagnosis, and a stable CKD group that did not show an increase >25% in plasma creatinine concentration. The cut‐point of 25% was based on the presumption that smaller changes could be caused by poor measurement precision rather than actual progression of azotemia.5, 8 Only stable cats with follow‐up of ≥12 months were included in our analysis. Magnesium was analyzed as a categorical variable divided on the lower and upper limits of the derived reference interval. Plasma FGF23 and PTH were log‐transformed before analysis. Variables associated with progressive disease with P <0.10 in univariable analysis entered multivariable regression. The final model was derived by manual backward elimination. Goodness of fit was assessed with Hosmer–Lemeshow test. Results are reported as OR (95% CI).
2.8. Interaction between phosphate and magnesium
A pre‐defined interaction between plasma tMg and phosphate status in association with survival was explored. Cats with IRIS stage 2 and 3 CKD were divided based on phosphate status according to IRIS phosphate target guidelines (International Renal Interest Society Guidelines: Treatment Recommendations for CKD in Cats (2015). http://www.iris-kidney.com/guidelines/recommendations.html), and by a median split of plasma tMg. This resulted in the following 4 categories: normophosphatemic‐lower magnesium (NP‐LM: normophosphatemic; tMg < 2.04 mg/dL), normophosphatemic‐higher magnesium (NP‐HM: normophosphatemic; tMg ≥ 2.04 mg/dL), hyperphosphatemic‐lower magnesium (HP‐LM: hyperphosphatemic; tMg < 2.04 mg/dL), and hyperphosphatemic‐higher magnesium (HP‐HM: hyperphosphatemic; tMg ≥ 2.04 mg/dL).
Statistical interaction between plasma tMg and phosphate in relation to survival was explored by comparing hazards of the 4 phosphate‐magnesium groups using univariable Cox regression. The NP‐HM group was chosen as the joint reference category of no exposure to calculate the relative excess risk due to interaction (RERI, ie, the difference between the expected risk and the observed risk; RERI = HRHP‐LM HRNP‐LM HRHP‐HM + 1) of the HP‐LM group using an available online tool (http://epinet.se/Epidemiologicaltools.htm).33
3. RESULTS
3.1. Determination of a 95% reference interval for plasma total magnesium concentration in older cats
The reference population consisted of 53 male cats (1 entire) and 67 female cats (1 entire). Cats were of the following breeds: domestic shorthair (n = 97), domestic longhair (n = 12), Burmese (n = 4), 2 each of Persian, British shorthair and Russian blue, and 1 British blue. Further characteristics can be found in Table 1. The distribution of plasma tMg was determined to be Gaussian, with a mean concentration of 2.15 (SD, 0.209) mg/dL, resulting in a 95% reference interval of 1.73–2.57 mg/dL (0.71–1.06 mmol/L). No correlation was observed between age and plasma tMg in the reference population (r, −0.09; P = 0.32).
Table 1.
Characteristics of 120 apparently healthy cats ≥ 9 years from which the reference interval for plasma total magnesium concentration was derived
| Variable (reference interval) | Median [25th, 75th Percentile] | n |
|---|---|---|
| Age (years) | 12.4 [11.1, 14.0] | 120 |
| Weight (kg) | 4.51 [3.81, 5.20] | 120 |
| Creatinine (0.23–2.00 mg/dL) | 1.45 [1.32, 1.65] | 120 |
| USG (≥1.035) | 1.048 [1.040, 1.058] | 80 |
| Phosphate (2.79–6.81 mg/dL) | 3.85 [3.28, 4.30] | 120 |
| Total calcium (8.2–11.8 mg/dL) | 9.8 [9.4, 10.2] | 120 |
| Total protein (6.0–8.0 g/dL) | 7.7 [7.3, 8.0] | 120 |
| Albumin (2.5–4.5 g/dL) | 3.3 [3.0, 3.4] | 120 |
| PCV (30%–45%) | 38 [35, 41] | 120 |
| Sodium (145–157 mEq/L) | 152 [152, 154] | 120 |
| Potassium (3.5–5.5 mEq/L) | 3.9 [3.7, 4.2] | 120 |
| Chloride (100–124 mEq/L) | 119 [117, 121] | 120 |
| SBP (<160 mm Hg) | 136 [120, 150] | 120 |
Abbreviations: PCV, packed cell volume; SBP, systolic blood pressure; USG, urine specific gravity.
3.2. Plasma total magnesium in cats with azotemic CKD
Between August 1999 and July 2013, a total of 517 cats were diagnosed with azotemic CKD, of which 96 cats were excluded for the following reasons: concurrent hyperthyroidism (n = 79), not meeting the study criteria for diagnosis of CKD (n = 16), or prednisolone administration (n = 1). Of the 421 eligible cats, 88 cats had no residual plasma sample available for measurement of tMg, 157 cats lacked baseline information on plasma FGF23 concentration, and 2 samples were grossly hemolyzed. Thus, 174 cats were enrolled in our study, some of which had been included in previous studies.5, 8 No significant differences were observed between baseline characteristics of the 174 included cats and of the 247 eligible cats that were excluded from analysis because of lack of a residual plasma sample or plasma FGF23 measurement (data not shown).
The study population consisted of 88 females (1 entire) and 86 males (3 entire). Domestic shorthair was the most common breed (n = 127), followed by domestic longhair (n = 20), Persian (n = 10), Burmese (n = 7), British shorthair (n = 2), Siamese (n = 2), and 1 each of Abyssinian, American shorthair, Chinchilla, Ocicat, Russian blue, and Tiffany. According to the IRIS staging system, 114 cats had stage 2, 50 cats stage 3, and 10 cats stage 4 CKD. Systemic hypertension was diagnosed in 37 cats (21%), 16 of which had their blood pressure normalized (<160 mm Hg) with PO amlodipine besylate, and 21 being new diagnoses. The study population was older than the group of apparently healthy cats from which the reference interval for plasma tMg was derived (mean, 14.4 years; SD, 3.2 versus 12.7 years; SD, 2.2, respectively).
3.3. Prevalence and factors associated with magnesium disorders
The median plasma tMg of the study population was 2.07 [1.87, 2.26] mg/dL (range, 1.29–5.79). Twenty of 174 cats, including 1 Persian and 1 British shorthair, were diagnosed with hypomagnesemia (prevalence, 12%; 95% CI, 7–17), and 11 cats, including 1 Persian, with hypermagnesemia (prevalence, 6%; 95% CI, 3–10). Baseline characteristics of cats with hypomagnesemia, normomagnesemia, and hypermagnesemia are shown in Table 2. Risk factors associated with magnesium disorders can be found in Table 3. Hypermagnesemia was predominantly observed in cats with IRIS stage 4 (Figure 1), but no multivariable analysis was performed because of the relatively low number of cases. Higher plasma ln[FGF23] (OR, 2.07; 95% CI, 1.48–2.90; P < .001) and a diagnosis of systemic hypertension (OR, 4.24; 95% CI, 1.41–12.78; P = .010) were independently associated with hypomagnesemia (Nagelkerke R 2, 0.30). The median plasma tMg of the subgroup of cats with a diagnosis of systemic hypertension was 1.97 [1.69, 2.14] mg/dL, compared with 2.09 [1.90, 2.29] mg/dL in the normotensive group (P = .004). No statistically significant difference in mean tMg was observed between cats treated with PO amlodipine besylate (mean, 1.99 mg/dL; SD, 0.408) and cats with newly diagnosed and thus untreated hypertension (mean, 1.87 mg/dL; SD, 0.310; P = .34).
Table 2.
Characteristics of cats with azotemic CKD grouped according to magnesium status
| Variable (reference interval) | Hypomagnesemic (n = 20) | Normomagnesemic (n = 143) | Hypermagnesemic (n = 11) | |||
|---|---|---|---|---|---|---|
| Total magnesium (1.73–2.57 mg/dL) | 1.57 [1.41, 1.66] | 20 | 2.07 [1.92, 2.24] | 143 | 2.87 [2.70, 3.60] | 11 |
| Age (years) | 16.6 [15.0, 18.2]a | 19 | 14.8 [12.0, 16.2] | 130 | 12.0 [9.8, 15.0]b | 11 |
| Weight (kg) | 2.98 [2.51, 3.56]a | 19 | 3.92 [3.14, 4.61]b | 142 | 3.63 [3.25, 4.20] | 9 |
| BCS (1–9) | 3 [3, 4] | 10 | 4 [3, 5] | 90 | 3 [3, 5] | 5 |
| Sex (male, n [%]) | 7 (35) | 20 | 72 (50) | 143 | 7 (64) | 11 |
| Albumin (2.5–4.5 g/dL) | 3.0 [2.8, 3.3] | 20 | 3.1 [2.9, 3.3] | 143 | 3.0 [2.9, 3.3] | 11 |
| Chloride (100–124 mEq/L) | 117 [116, 119] | 20 | 118 [116, 120] | 143 | 117 [114, 122] | 11 |
| Cholesterol (85–154 mg/dL) | 234 [190, 277] | 20 | 199 [158, 246] | 143 | 212 [170, 247] | 11 |
| Creatinine (0.23–2.00 mg/dL) | 2.85 [2.32, 4.03]a | 20 | 2.48 [2.25, 2.96]a | 143 | 4.82 [2.65, 5.47]b | 11 |
| FGF23 (56–700 pg/mL) | 4950 [1931, 15893]a | 20 | 637 [351, 1941]b | 143 | 2658 [684, 8582] | 11 |
| FGF23 excess (n [%]) | 18 (90)a | 20 | 68 (48)b | 143 | 8 (73) | 11 |
| Globulin (2.5–4.5 g/dL) | 4.4 [4.1, 4.9]a | 20 | 4.7 [4.3, 5.3] | 143 | 4.8 [4.5, 6.2]b | 11 |
| PCV (30%–45%) | 30 [24, 34] | 20 | 34 [30, 37] | 141 | 32 [23, 34] | 11 |
| Phosphate (2.79–6.81 mg/dL) | 5.51 [4.47, 6.93]a | 20 | 4.43 [3.75, 5.39]b | 143 | 6.16 [4.46, 9.54]a | 11 |
| Hyperphosphatemia (% [n]) | 14 (70) | 20 | 64 (45) | 143 | 7 (64) | 11 |
| Potassium (3.5–5.5 mEq/L) | 3.9 [3.7, 4.4] | 20 | 4.1 [3.7, 4.3] | 143 | 4.0 [3.4, 4.8] | 11 |
| Hypokalemia (n [%]) | 2 (10) | 20 | 17 (12) | 143 | 3 (27) | 11 |
| Hyperkalemia (n [%]) | 0 (0) | 20 | 0 (0) | 143 | 1 (9) | 11 |
| PTH (2.6–17.6 pg/mL) | 46.3 [12.5, 93.0]a | 17 | 15.2 [6.5, 31.2]b | 138 | 25.0 [11.7, 81.9] | 10 |
| SRHPT (n [%]) | 12 (71) | 17 | 67 (49) | 138 | 6 (60) | 10 |
| SBP (<160 mm Hg) | 153 [134, 163] | 20 | 142 [128, 156] | 143 | 136 [128, 150] | 11 |
| Hypertension (n [%]) | 9 (45)a | 20 | 26 (18)b | 143 | 2 (18) | 11 |
| Sodium (145–157 mEq/L) | 154 [153, 155] | 20 | 153 [152, 155] | 143 | 151 [150, 156] | 11 |
| Total calcium (8.2–11.8 mg/dL) | 10.7 [10.1, 11.0]a | 20 | 10.2 [9.7, 10.5] | 143 | 9.6 [9.4, 10.4]b | 11 |
| Hypocalcemia (n, [%]) | 0 (0) | 20 | 1 (1) | 143 | 0 (0) | 11 |
| Hypercalcemia (n, [%]) | 1 (5) | 20 | 2 (1) | 143 | 0 (0) | 11 |
| Total protein (6.0–8.0 g/dL) | 7.6 [7.1, 7.9]a | 20 | 7.8 [7.5, 8.3]b | 143 | 8.2 [7.6, 9.1]b | 11 |
| UPC (<0.20) | 0.31 [0.27, 0.61]a | 13 | 0.17 [0.12, 0.32]b | 115 | 0.50 [0.23, 1.19]a | 9 |
| UPC <0.20 (n [%]) | 1 (8)a | 13 | 70 (61)b | 115 | 1 (11)a | 9 |
| UPC >0.40 (n [%]) | 5 (39) | 13 | 21 (18)a | 115 | 5 (56)b | 9 |
| USG (≥1.035) | 1.016 [1.014, 1.020] | 19 | 1.018 [1.016, 1.021] | 136 | 1.016 [1.014, 1.018] | 10 |
| UTI (n, [%]) | 1 (5) | 19 | 15 (11) | 136 | 0 (0) | 10 |
| Progressive CKD (n [%]) | 7 (88)a | 8 | 19 (28)b | 67 | 3 (75) | 4 |
| Survival time (days) | 147 [52, 328]a | 19 | 559 [205, 879]b | 120 | 125 [50, 423]a | 11 |
| Follow‐up (days) | 152 [53, 336]a | 20 | 546 [207, 890]b | 143 | 125 [50, 423]a | 11 |
| Year of diagnosis | 2008 [2004, 2011] | 20 | 2009 [2007, 2011] | 143 | 2008 [2003, 2012] | 11 |
Data presented as median [25th, 75th percentile] or prevalence (n [%]). Rows bearing a different superscript letter are significantly different from one another.
Abbreviations: BCS, body condition score; FGF23, fibroblast growth factor 23; PCV, packed cell volume; PTH, parathyroid hormone; SRHPT, secondary renal hyperparathyroidism; SBP, systolic blood pressure; UPC, urine protein to creatinine ratio; USG, urine specific gravity; UTI, bacterial urinary tract infection.
Table 3.
Univariable binary logistic regression results identifying risk factors for hypomagnesemia (n = 20) and hypermagnesemia (n = 11) in 174 cats with azotemic CKD
| Univariable analysis | n | OR (95% CI) | P |
|---|---|---|---|
| Hypomagnesemia | |||
| ln[FGF23] (pg/mL) | 163 | 1.99 (1.46–2.73) | <.001 |
| Weight (kg) | 161 | 0.25 (0.11–0.55) | .001 |
| Diagnosis of hypertension | 163 | 3.68 (1.38–9.79) | .009 |
| Phosphate (mg/dL) | 163 | 1.26 (1.05–1.51) | .013 |
| ln[PTH] (pg/mL) | 155 | 1.77 (1.13–2.77) | .013 |
| PCV (%) | 161 | 0.92 (0.85–0.99) | .028 |
| Creatinine (mg/dL) | 163 | 1.48 (1.04–2.12) | .032 |
| Age (years) | 149 | 1.20 (1.01–1.42) | .033 |
| Total calcium (mg/dL) | 163 | 1.86 (1.03–3.38) | .041 |
| Hypermagnesemia | |||
| Creatinine (mg/dL) | 154 | 2.22 (1.36–2.63) | .001 |
| UPC | 124 | 2.72 (1.37–5.37) | .004 |
| Phosphate (mg/dL) | 154 | 1.33 (1.09–1.61) | .006 |
| PCV (%) | 152 | 0.90 (0.82–0.99) | .029 |
| Age (years) | 141 | 0.81 (0.66–0.99) | .044 |
Plasma FGF23 concentration (or, 2.07; 95% CI, 1.48–2.90; P < .001) and a diagnosis of systemic hypertension (or, 4.24; 95% CI, 1.41–12.78; P = .010) remained independent risk factors for hypomagnesemia in multivariable analysis (n = 163). No multivariable regression was performed for hypermagnesemia.
Abbreviations: 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; OR, odds ratio; PCV, packed cell volume; PTH, parathyroid hormone; UPC, urine protein to creatinine ratio.
Figure 1.
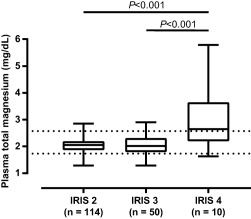
Plasma total magnesium concentration in cats with IRIS stages 2–4 CKD. Significantly higher plasma magnesium concentrations were observed in cats with IRIS stage 4 CKD (mean, 3.01 mg/dL; SD, 1.206; P < .001) compared with cats with IRIS stage 2 (mean, 2.04 mg/dL; SD, 0.261) and 3 CKD (mean, 2.04 mg/dL; SD, 0.361). The prevalence (95% CI) of hypomagnesemia was 9% (4–14), 18% (7–29), and 10% (0–27), respectively, in IRIS stages 2, 3, and 4. The prevalence of hypermagnesemia was significantly higher in IRIS stage 4 (50%; 95% CI, 19–81; P < .001) compared with IRIS stages 2 (3%; 95% CI, 0–6) and 3 (6%; 95% CI, 0–13). The boxes represent medians with 25th and 75th percentiles, the whiskers represent ranges. Dotted lines mark the lower and upper limits of the reference interval for plasma total magnesium (1.73‐2.57 mg/dL)
3.4. Association of plasma total magnesium with plasma FGF23 and other clinicopathological variables
Hypomagnesemic cats had higher plasma FGF23 than normomagnesemic cats within each IRIS stage (Figure 2). No simple correlation was evident between plasma tMg and ln[FGF23] (r, −0.06; P = .43), but controlling for plasma creatinine and phosphate resulted in a significant inverse correlation (partial r, −0.50; P < .001). The final multivariable model indicated that plasma FGF23 was an independent predictor of plasma tMg in all 3 IRIS stages, with the strongest effect in stage 4 CKD (Table 4).
Figure 2.
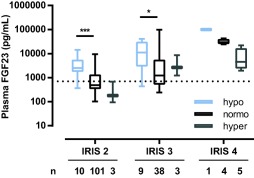
Plasma FGF23 concentrations in cats with IRIS stage 2–4 CKD subdivided by magnesium status (tMg reference interval, 1.73‐2.57 mg/dL). Cats with hypomagnesemia had significantly higher plasma FGF23 compared with normomagnesemic cats within each stage. The boxes represent medians with 25th and 75th percentiles, the whiskers represent ranges. The dotted line marks the upper limit of the reference interval for plasma FGF23 (700 pg/mL). *, P < .05; ***, P < .001
Table 4.
General linear model to identify predictors of plasma total magnesium concentration (mg/dL)
| Univariable analysis a | Multivariable model (n = 160) | |||
|---|---|---|---|---|
| Variable | β (95% CI) | n | β (95% CI) | n |
| IRIS stage | ||||
| IRIS 2 | 2.04 (1.97 to 2.11) | 114 | 2.83 (1.97 to 3.70) | 106 |
| IRIS 3 | 2.04 (1.93 to 2.16) | 50 | 2.90 (1.59 to 4.20) | 46 |
| IRIS 4 | 3.01 (2.76 to 3.26) | 10 | 10.80 (7.87 to 13.72) | 8 |
| Age (years) | −0.02 (−0.04 to −0.01) | 160 | −0.03 (−0.04 to −0.01) | |
| Weight (kg) | 0.09 (0.02 to 0.15) | 170 | ||
| ln[PTH] (pg/dL) | −0.05 (−0.10 to 0.00) | 165 | ||
| Chloride (mEq/L) | 0.01 (−0.00 to 0.03) | 174 | ||
| Hypertension | −0.19 (−0.33 to −0.04) | 174 | ||
| ln[Creatinine] (mg/dL) | ||||
| IRIS 2 | 0.17 (−0.56 to 0.90) | 114 | 0.63 (0.04 to 1.22) | |
| IRIS 3 | 0.27 (−0.33 to 0.87) | 50 | 0.68 (0.08 to 1.29) | |
| IRIS 4 | 1.57 (1.05 to 2.08) | 10 | 1.48 (1.05 to 1.90) | |
| ln[FGF23] (pg/mL) | ||||
| IRIS 2 | −0.10 (−0.16 to −0.05) | 114 | −0.11 (−0.16 to −0.06) | |
| IRIS 3 | −0.06 (−0.11 to −0.00) | 50 | −0.10 (−0.16 to −0.03) | |
| IRIS 4 | −0.76 (−0.93 to −0.60) | 10 | −0.44 (−0.65 to −0.24) | |
| Albumin (g/dL) | ||||
| IRIS 2 | 0.10 (−0.11 to 0.33) | 114 | ||
| IRIS 3 | 0.27 (−0.02 to 0.57) | 50 | ||
| IRIS 4 | 2.67 (1.89 to 3.45) | 10 | ||
| Potassium (mEq/L) | ||||
| IRIS 2 | 0.08 (−0.06 to 0.23) | 114 | ||
| IRIS 3 | 0.02 (−0.17 to 0.21) | 50 | ||
| IRIS 4 | 0.84 (0.57 to 1.12) | 10 | ||
| Total calcium (mg/dL) | ||||
| IRIS 2 | −0.05 (−0.15 to 0.04) | 114 | −0.02 (−0.10 to 0.06) | |
| IRIS 3 | −0.06 (−0.20 to 0.07) | 50 | −0.06 (−0.17 to 0.05) | |
| IRIS 4 | −0.97 (−1.32 to −0.63) | 10 | −0.59 (−0.97 to −0.21) | |
R 2 multivariable model = 0.69.
aAll variables are accounted for IRIS stage.
Abbreviations: β, regression coefficient; 95% CI, 95% confidence interval; IRIS, international renal interest society; ln[PTH], log‐transformed plasma parathyroid hormone concentration; ln[FGF23], log‐transformed plasma fibroblast growth factor 23 concentration.
3.5. Association of plasma total magnesium with survival
During the total follow‐up period of 270.4 patient‐years (median, 1.3 [0.5, 2.3] years), 150 cats died, 20 were lost to follow‐up, and 4 survived beyond 1st July 2016. Risk of death in the first 12 months after diagnosis of azotemic CKD was 43% (72/167) for the whole population, 35% (48/136) for cats with normomagnesemia at baseline, 80% (16/20) for cats with hypomagnesemia, and 73% (8/11) for those with hypermagnesemia. The incidence rate of all‐cause mortality was 0.56 per patient‐year for all cats, 0.48 per patient‐year for cats with normomagnesemia, and 1.34 per patient‐year for cats with hypomagnesemia and cats with hypermagnesemia at diagnosis of CKD.
Baseline characteristics of the three magnesium categories can be found in Table 2. Censoring occurred in 16% (23/143) of normomagnesemic cats, 5% (1/20) of hypomagnesemic cats, and none of the hypermagnesemic cats. Univariable survival analysis indicated that hypomagnesemia and hypermagnesemia were associated with increased risk of death (Table 5 and Figure 3). After adjustment for confounders, hypomagnesemia remained an independent predictor of death, but the association between hypermagnesemia and risk of death lost significance. No statistically significant differences were observed between baseline characteristics of cats incorporated in the final regression model (n = 122) and those of cats omitted because of missing information (n = 52, data not shown).
Table 5.
Time‐invariant cox regression results identifying baseline predictors of mortality in cats with azotemic CKD
| Variables | n | HR | 95% CI | P |
|---|---|---|---|---|
| A. Univariable results | ||||
| Normomagnesemia | 143 | <.001 | ||
| Hypomagnesemia | 20 | 2.92 | 1.78–4.82 | <.001 |
| Hypermagnesemia | 11 | 2.88 | 1.54–5.38 | .001 |
| FGF23 (<460 pg/mL) | 56 | <.001 | ||
| 460–1800 pg/mL | 58 | 1.12 | 0.75–1.69 | .58 |
| >1800 pg/mL | 60 | 2.69 | 1.80–4.01 | <.001 |
| Age (years) | 160 | 1.09 | 1.03–1.16 | .003 |
| Weight (≤3.20 kg) | 57 | <.001 | ||
| 3.21–4.15 kg | 57 | 0.57 | 0.38–0.84 | .005 |
| ≥4.16 kg | 56 | 0.40 | 0.26–0.60 | <.001 |
| BCS (ideal weight) | 26 | .015 | ||
| Underweight | 65 | 2.03 | 1.23–3.37 | .006 |
| Overweight | 14 | 1.25 | 0.60–2.61 | .55 |
| Albumin (g/dL) | 174 | 0.38 | 0.22–0.67 | .001 |
| Creatinine (mg/dL) | 174 | 1.48 | 1.32–1.65 | <.001 |
| PCV (%) | 172 | 0.91 | 0.88–0.94 | <.001 |
| Phosphate (<4.00 mg/dL) | 57 | .001 | ||
| 4.00–5.26 mg/dL | 58 | 1.15 | 0.77–1.72 | .49 |
| ≥5.27 mg/dL | 59 | 2.09 | 1.41–3.10 | <.001 |
| ln[PTH] (pg/mL) | 164 | 1.17 | 1.01–1.36 | .043 |
| Normotensive cats | 137 | |||
| Diagnosis of hypertension | 37 | 1.51 | 1.03–2.23 | .036 |
| USG (≤1.016) | 66 | .031 | ||
| 1.017–1.019 | 45 | 0.68 | 0.45–1.03 | .068 |
| ≥1.020 | 54 | 0.62 | 0.42–0.91 | .014 |
| UPC | 137 | 1.74 | 1.34–2.25 | <.001 |
| Interactions with magnesium status | ||||
| Creatinine (mg/dL) | .019 | |||
| Normomagnesemia | 143 | 1.82 | 1.47–2.24 | <.001 |
| Hypomagnesemia | 20 | 1.23 | 0.97–1.56 | .092 |
| Hypermagnesemia | 11 | 1.69 | 1.09–2.65 | .020 |
| UPC | .030 | |||
| Normomagnesemia | 115 | 1.62 | 1.18–2.23 | .003 |
| Hypomagnesemia | 13 | 1.30 | 0.54–3.12 | .56 |
| Hypermagnesemia | 9 | 6.13 | 1.13–33.29 | .036 |
| Multivariable model (n = 122) | ||||
| Normomagnesemia | 101 | .017 | ||
| Hypomagnesemia | 12 | 2.74 | 1.35–5.55 | .005 |
| Hypermagnesemia | 9 | 1.66 | 0.74–3.70 | .22 |
| Age (years) | 122 | 1.18 | 1.08–1.28 | <.001 |
| Creatinine (mg/dL) | 122 | 1.29 | 1.12–1.49 | .001 |
| PCV (%) | 122 | 0.92 | 0.89–0.96 | <.001 |
| UPC | 122 | 2.28 | 1.45–3.60 | <.001 |
| B. Phosphate‐magnesium groups | ||||
| NP‐HM | 46 | .025 | ||
| NP‐LM | 42 | 0.97 | 0.61–1.53 | .89 |
| HP‐HM | 41 | 1.14 | 0.71–1.80 | .59 |
| HP‐LM | 35 | 1.90 | 1.19–3.04 | .008 |
A. Univariable and multivariable regression results of the main analysis. B. Univariable results of the subanalysis examining the prespecified interaction between phosphate and magnesium.
Abbreviations: 95% CI, 95% confidence interval; BCS, body condition score; FGF23, fibroblast growth factor 23; HM, higher plasma magnesium; HP, hyperphosphatemic; HR, hazard ratio; LM, lower plasma magnesium; PCV, packed cell volume; PTH, parathyroid hormone; NP, normophosphatemic; UPC, urine protein to creatinine ratio; USG, urine specific gravity.
Figure 3.
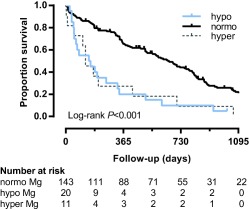
Kaplan‐Meier curve illustrating survival in cats with azotemic CKD grouped by magnesium status. Cats with hypomagnesemia (blue curve; 1/20 censored; HR, 2.92; 95% CI, 1.78‐4.82; P < .001) and hypermagnesemia (gray curve; 0/11 censored; HR, 2.88; 95% CI, 1.54‐5.38; P = .001) at diagnosis of azotemic CKD were at increased risk of death compared with normomagnesemic cats (black curve; 23/143 censored). No significant difference in survival was observed between hypomagnesemic and hypermagnesemic cats in univariable analysis (P = .95). Hypomagnesemia remained an independent predictor of mortality in multivariable analysis (HR, 2.74; 95% CI, 1.35‐5.55; P = .005), but the association between hypermagnesemia and mortality lost significance (HR, 1.66; 95% CI, 0.74‐3.70; P = .22). Censored cases are ticked
Treated as a continuous variable in the fully adjusted model, plasma tMg was inversely associated with risk of death in cats with plasma tMg <2.07 mg/dL (HR, 0.04; 95% CI, 0.01–0.27; P = .001; n = 57; mean tMg, 1.83 mg/dL). The association was nonlinear and nonsignificant in cats with tMg ≥2.07 mg/dL (HR, 0.67; 95% CI, 0.24–1.85; P = .44; n = 65; mean tMg, 2.37 mg/dL). In the highest quartile, however, tMg was significantly associated with mortality (tMg ≥2.26 mg/dL: HR, 0.12; 95% CI, 0.02–0.76; P = .025; n = 31, mean tMg, 2.62 mg/dL).
3.6. Association of plasma total magnesium with progression of CKD
Seventy‐nine cats had sufficient follow‐up data available to be included in the progression analysis, of which 29 cats (37%) showed progression of CKD within the first 12 months of diagnosis. Median plasma tMg did not differ significantly between groups (stable: 2.03 [1.89, 2.21] mg/dL, progressive: 2.04 [1.75, 2.21] mg/dL; P = .60), but a significantly higher proportion of hypomagnesemic cats had progressive CKD (P = .001; Table 2). Hypomagnesemia was associated with increased odds of progressive disease in univariable analysis, but the effect of magnesium lost significance after adjustment for additional variables. Only higher plasma FGF23 remained a significant predictor of progressive CKD in the final logistic regression model (Nagelkerke R 2, .21; Table 6).
Table 6.
Univariable binary logistic regression results identifying predictors of progressive CKD within the first 12 months of diagnosis of azotemic CKD in cats
| Univariable analysis | Stable (n = 50) | Progressive (n = 29) | n | OR (95% CI) | P |
|---|---|---|---|---|---|
| Normomagnesemia | 48 (72) | 19 (28) | 67 | .010 | |
| Hypomagnesemia | 1 (12) | 7 (88) | 8 | 17.68 (2.04–153.59) | .009 |
| Hypermagnesemia | 1 (25) | 3 (75) | 4 | 7.58 (0.74–77.48) | .088 |
| FGF23 (pg/mL) | 459 [334, 1037] | 1944 [575, 5761] | 79 | 1.90 (1.29–2.80) | .001 |
| PCV (%) | 34 [32, 37] | 30 [25, 34] | 79 | 0.87 (0.79–0.96) | .004 |
| Creatinine (mg/dL) | 2.37 [2.23, 2.80] | 2.69 [2.39, 3.77] | 79 | 2.65 (1.26–5.56) | .010 |
| Phosphate (mg/dL) | 4.38 [3.50, 5.09] | 4.95 [3.95, 6.05] | 79 | 1.33 (1.01–1.74) | .043 |
| Albumin (g/dL) | 3.2 [3.1, 3.3] | 3.0 [2.8, 3.2] | 79 | 0.19 (0.04–0.95) | .043 |
Only log‐transformed plasma FGF23 concentration remained an independent risk factor for progression with multivariable regression. Group characteristics are reported as number (%) or median [25th, 75th percentiles].
Abbreviations: 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; OR, odds ratio; PCV, packed cell volume.
3.7. Interaction between phosphate and magnesium
In the subgroup analysis of NP and HP cats (based on IRIS targets for plasma phosphate for each stage) with IRIS stage 2 and 3 CKD, plasma FGF23 concentration was significantly higher in cats with lower plasma tMg compared to cats with higher plasma tMg (Figure 4). In a general linear model adjusted for IRIS stage, ln[FGF23] was negatively associated with plasma tMg both in normophosphatemic cats (β, −0.11; 95% CI, −0.17 to −0.05; P = .001) and hyperphosphatemic cats (β, −0.10; 95% CI, −0.15 to −0.05; P < .001).
Figure 4.
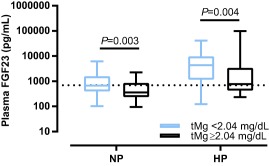
Plasma FGF23 concentrations of NP and HP cats with IRIS stage 2 and 3 CKD subdivided by the median plasma tMg concentration (2.04 mg/dL). Plasma FGF23 was significantly higher in cats with tMg below the median, both in normophosphatemic (P = .003) and hyperphospatemic cats (P = .004). The boxes represent medians with 25th and 75th percentiles, the whiskers represent ranges. The dotted line marks the upper limit of the reference interval for plasma FGF23 (700 pg/mL)
Cats that were hyperphosphatemic for IRIS stage at diagnosis of CKD (n = 88) had increased risk of death (HR, 1.44; 95% CI, 1.03–2.01; P = .034) compared with normophosphatemic cats (n = 76). However, taking plasma tMg into account, a significantly increased risk of death was observed only in hyperphosphatemic cats with plasma tMg below the median (HP‐LM = reference group; P = .025, NP‐HM: HR, 0.53; 95% CI, 0.33–0.84; P = .008, NP‐LM: HR, 0.51; 95% CI, 0.31–0.83; P = .007, HP‐HM: HR, 0.60; 95% CI, 0.36–0.99; P = .043). Compared with the NP‐HM group as the joint reference category of no exposure, departure from additivity was observed with a relative excess risk of 0.79 (95% CI, −0.05–1.65) in the HP‐LM group, suggesting interaction might exist between low magnesium and high phosphate in relation to survival, although the 95% CI included zero (Table 5).
No statistically significant difference in risk of progression of azotemia was observed between hyperphosphatemic and normophosphatemic cats (HP versus NP: OR, 1.62; 95% CI, 0.63–4.15; P = .32). Therefore, the effect of joint exposure of plasma tMg and phosphate in relation to progressive CKD was not explored.
4. DISCUSSION
Results from our observational cohort demonstrate an inverse relationship between plasma tMg and plasma FGF23 concentrations in cats with azotemic CKD. A significant independent association between hypomagnesemia and increased risk of death was observed. Insufficient evidence was found for an independent association between magnesium status and risk of progressive CKD. In additional analyses, the risk of death associated with hyperphosphatemia appeared mitigated by higher plasma tMg, and a possible link between hypomagnesemia and systemic hypertension was identified.
Hypomagnesemia in CKD is thought to be secondary to impaired intestinal absorption or increased renal excretion of magnesium, with depletion of bone and muscle reserves,34, 35, 36, 37 whilst hypermagnesemia results from incapacity of the kidneys to filter sufficient magnesium.37, 38 Hypermagnesemia was found mostly in cats with severe renal dysfunction in our study (5 of 10 cats with IRIS Stage 4 CKD), and the distribution of magnesium disorders across the different stages of CKD was comparable to those reported before in a smaller number of cats.3 However, the prevalences of hypomagnesemia and hypermagnesemia were possibly underestimated in our study, because these frequencies were assessed on a single time point rather than in a given time interval, whilst cats with these disorders were characterized by higher risks of death. Missing information on muscle mass necessitated IRIS stage classification based solely on plasma creatinine concentration, and consequently the severity of CKD could have been underestimated in some cats.
Hypomagnesemia was independently associated with systemic hypertension in cats, a relationship that is well‐known in human medicine.36, 39, 40, 41, 42, 43, 44 Magnesium plays an active role in vascular resistance via various mechanisms such as regulation of intracellular calcium concentration, nitric oxide production, and vascular calcification,45, 46, 47, 48, 49, 50, 51, 52, 53, 54 and in dogs a decrease in vascular resistance and SBP was observed after magnesium infusion.55 The difference in plasma tMg between normotensive cats and cats with a (previous) diagnosis of systemic hypertension, however, was small, and therefore not directly clinically relevant. Increased plasma aldosterone is commonly observed in azotemic cats with systemic hypertension and not influenced by treatment with amlodipine besylate.56, 57 It could be a possible link between hypomagnesemia and hypertension, because aldosterone stimulates urinary magnesium excretion58, 59 whilst magnesium inhibits aldosterone release.60, 61 No information on plasma aldosterone concentration was available for cats included in our study.
An inverse association of plasma tMg with FGF23 was found in our population of cats with CKD, and has previously been identified in human CKD patients on hemodialysis.29 Rodent studies suggest that circulating FGF23 is influenced by dietary magnesium intake,30, 31, 62 and lower serum FGF23 concentrations were observed in hemodialysis‐patients receiving magnesium‐containing laxatives or phosphate binders in comparison to patients not receiving PO magnesium.29, 63 However, no significant reduction in FGF23 concentration was reported in a study examining the effect of oral magnesium supplementation on serum calcification propensity in human CKD stage 3 and 4 patients.64 The underlying mechanisms of the relationship between magnesium and FGF23 remain to be elucidated, but it could be hypothesized that FGF23 has an effect on renal magnesium handling, as it was also shown to regulate tubular phosphate,65, 66 calcium,67 and sodium68 reabsorption. Aldosterone stimulates Fgf23 (mRNA) expression by osteoblasts,69 so alternatively increased plasma aldosterone, either as the cause of or secondary to hypomagnesemia, could contribute to higher circulating FGF23.
Hypomagnesemia at diagnosis of azotemic CKD was an independent predictor of death in cats. No previous survival studies in cats with CKD assessed the effect of magnesium status,5, 6, 7 but both hypomagnesemia and hypermagnesemia were associated with decreased survival in cats hospitalized in an intensive care unit.70 Multiple observational studies in human CKD patients report a link between hypomagnesemia and increased mortality, both in hemodialysis‐patients25, 28 and patients with nondialysis‐dependent CKD.24, 26 Moreover, the risk of death associated with hyperphosphatemia appears modified by serum tMg in hemodialysis‐patients.27 Hyperphosphatemia is a well‐recognized risk factor in feline CKD,6, 7 and our results might suggest that higher tMg mitigates the risk of death associated with hyperphosphatemia, as a significantly increased risk was observed only in HP cats with plasma tMg below the median value. A possible explanation could be an inhibitory role of magnesium on phosphate‐induced vascular calcification,21, 71 although only scant reports exist on soft tissue and vascular calcification in cats with CKD.2, 72, 73 In addition, both phosphate and magnesium could influence plasma FGF23, which has been identified as an important prognostic factor in cats with CKD.5 Plasma FGF23 was not a significant independent predictor of survival in the analysis presented here. Adjustment for additional variables in our study and differences in grouping of variables could explain this discrepancy. Baseline FGF23 was not included in the above‐mentioned survival models for humans with CKD.
The higher risk of death associated with hypermagnesemia in cats was lost after adjustment for additional variables and was possibly caused by its predominance in end‐stage CKD. It must be noted that this result was based on a low number of observations, resulting in a wide 95% confidence interval.
Magnesium has been shown to suppress phosphate‐induced damage to murine proximal tubular cells,23 and to reduce the degree of renal function decline associated with hyperphosphatemia in human CKD patients.23 Hypomagnesemia was a risk factor for progression of azotemia in our cats, but, similar to humans,24 the association was lost in multivariable analysis. Our analysis could have been impacted by the short survival time of cats with magnesium disturbances, as demonstrating progressive increases in plasma creatinine is more difficult when the follow‐up period is short. Moreover, hypomagnesemia might be a predictor for progression to end‐stage disease only in humans with diabetic nephropathy, but not in patients with nondiabetic CKD.74, 75 However, diabetes was not associated with risk of progression in another cohort of humans with CKD,24, 75, 76 in which use of diuretics was identified as a confounder instead. None of the cats in the present study had diabetes mellitus or were administered diuretics. All mentioned models examining the relation between serum magnesium and survival in human CKD were adjusted for concurrent diabetes mellitus.24, 25, 26, 27, 28
Although the relationship between hyperphosphatemia and renal fibrosis is well‐known in cats with CKD,8, 77, 78, 79 no evidence for a significant independent effect of plasma phosphate on progression of azotemia was found in the present study. Similar to the result from a previous study,5 plasma FGF23 was a positive confounder for this relationship. Moreover, the relatively low number of progressive cases might have led to insufficient statistical power to identify a significant association.
The effects of hypomagnesemia and hypermagnesemia on progression and survival might have been underestimated because of misclassification bias. Firstly, grouping of cats in different magnesium categories based on a single baseline measurement could have introduced regression dilution bias. Baseline magnesium concentration is lower in human patients that will develop hypomagnesemia during the course of their CKD,24 but in what manner plasma magnesium changes over time in cats with CKD is unknown. Secondly, tMg consist of 3 fractions: ionized, protein‐bound, and complexed magnesium.19 Ionized magnesium status was overestimated by tMg in cats with renal transplants and in cats with diabetes mellitus.80, 81 Thus, cats with ionized hypomagnesemia could have been included in the normomagnesemic category, which would have introduced exposure identification bias if the effects observed with low magnesium are because of ionized rather than total magnesium status. However, only 1% of body magnesium is located in the extracellular fluid and no consensus exists on whether measurement of tMg or biologically active ionized magnesium best represents magnesium status, nor is there good agreement between these methods.36, 37, 82
Although multivariable analyses were performed, the possibility of residual confounding cannot be eliminated, and incomplete information for muscle mass score, vitamin D‐metabolites, blood gases, bone parameters, and plasma aldosterone concentration prohibited investigation of relationships between magnesium and these variables. Multiple observations were made on a relatively small cohort of cats, and our findings therefore require validation by other studies in different populations of cats. Sequential measurements of plasma tMg would allow longitudinal analysis of the associations explored in our study and would especially benefit assessment of the relationship between plasma tMg and progression of azotemia. Further studies are warranted to explore possible relationships of magnesium with plasma aldosterone concentration, renal osteodystrophy and vascular calcification in cats.
Our study identified low plasma tMg to be associated with higher plasma FGF23 concentrations and reduced survival in cats with CKD, which is in agreement with results from studies exploring the relationships of tMg with FGF23 and survival in humans with CKD.24, 26, 28, 29, 63 These observations were made on client‐owned cats from first opinion practice with naturally occurring CKD and should be relevant to other populations of pet cats. Our results suggest that plasma tMg should be added to the routine plasma biochemistry panel assessed in cats with CKD, as this analyte adds prognostic information and helps to identify cats with marked bone‐mineral disorders. The observational design of our study does not allow conclusions on causation, and clinical trials could help investigate if magnesium is a modifiable risk factor, and if our findings could lead to the development of new management strategies in CKD‐MBD in cats.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest. Henk van den Broek received PhD studentship grant funding from Royal Canin. Jonathan Elliot was a consultant in Elanco Ltd, CEVA Animal Health Ltd, Boehringer Ingelheim Ltd, Bayer Animal Health, Orion Incorp, Idexx Ltd, Nextvet Ltd, Waltham Centre for Pet Nutrition. Received grant funding from Royal Canin Ltd, Elanco Ltd, Waltham Centre for Pet Nutrition, Zoetis Ltd, CEVA Animal Health. Member of the International Renal Interest Society which receives a grant from Elanco Ltd. Rosanne Jepson received funding from PetPlan, Feline Foundation for Renal Research, RVC Internal Grant, PetSavers. Consultancy agreements: Boehringer Ingelheim, Merial. Speaking honoraria: Boehringer Ingelheim and Hills Pet Nutrition. Ruby Chang declared no conflicts of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Cats enrolled in this study were part of a larger observational cohort for which approval of the Ethics and Welfare Committee of the Royal Veterinary College had been granted.
ACKNOWLEDGMENTS
This study was performed at the Royal Veterinary College, London, UK and supported by a grant from Royal Canin SAS, Aimargues, France. The Renal Research Clinic at the Royal Veterinary College acknowledges support from Royal Canin for its research on chronic kidney disease‐mineral and bone disorder in cats.
Van den Broek DHN, Chang Y‐M, Elliott J, Jepson RE. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J Vet Intern Med. 2018;1359:1–1371. 10.1111/jvim.15141
Funding information Royal Canin
REFERENCES
- 1. Lulich JP, Osborne CA, Obrien TD, Polzin DJ. Feline renal‐failure ‐ questions, answers, questions. Compen Contin Educ Pract Vet. 1992;14:127–152. [Google Scholar]
- 2. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc. 1987;190:1196–1202. [PubMed] [Google Scholar]
- 3. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:108–116. [DOI] [PubMed] [Google Scholar]
- 4. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27:234–241. [DOI] [PubMed] [Google Scholar]
- 5. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. King JN, Tasker S, Gunn‐Moore DA; BENRIC (benazepril in renal insufficiency in cats) Study Group . Prognostic factors in cats with chronic kidney disease. J Vet Intern Med. 2007;21:906–916. [PubMed] [Google Scholar]
- 7. Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med. 2008;22:1111–1117. [DOI] [PubMed] [Google Scholar]
- 8. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275–281. [DOI] [PubMed] [Google Scholar]
- 9. Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD‐related mineral and bone disorder. Kidney Int Suppl. 2011;79:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geddes RF, Finch NC, Syme HM, et al. The role of phosphorus in the pathophysiology of chronic kidney disease. J Vet Emerg Crit Care. 2013;23:122–133. [DOI] [PubMed] [Google Scholar]
- 12. Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36:51–62. [DOI] [PubMed] [Google Scholar]
- 13. Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2006;69:1945–1953. [DOI] [PubMed] [Google Scholar]
- 14. Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40:62–70. [DOI] [PubMed] [Google Scholar]
- 15. Elliott J, Rawlings JM, Markwell PJ, Barber PJ. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract. 2000;41:235–242. [DOI] [PubMed] [Google Scholar]
- 16. Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc. 2006;229:949–957. [DOI] [PubMed] [Google Scholar]
- 17. Plantinga EA, Everts H, Kastelein AM, Beynen AC. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec. 2005;157:185–187. [DOI] [PubMed] [Google Scholar]
- 18. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med. 2013;27:1354–1361. [DOI] [PubMed] [Google Scholar]
- 19. Jahnen‐Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5:i3–i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakaguchi Y, Hamano T, Nakano C, et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One. 2016;11:e0163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louvet L, Buchel J, Steppan S, Passlick‐Deetjen J, Massy ZA. Magnesium prevents phosphate‐induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Laecke S, Marechal C, Verbeke F, et al. The relation between hypomagnesaemia and vascular stiffness in renal transplant recipients. Nephrol Dial Transplant. 2011;26:2362–2369. [DOI] [PubMed] [Google Scholar]
- 23. Sakaguchi Y, Iwatani H, Hamano T, et al. Magnesium modifies the association between serum phosphate and the risk of progression to end‐stage kidney disease in patients with non‐diabetic chronic kidney disease. Kidney Int. 2015;88(4):833–842. [DOI] [PubMed] [Google Scholar]
- 24. Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med. 2013;126:825–831. [DOI] [PubMed] [Google Scholar]
- 25. Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non‐cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181. [DOI] [PubMed] [Google Scholar]
- 26. Kanbay M, Yilmaz MI, Apetrii M, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36:228–237. [DOI] [PubMed] [Google Scholar]
- 27. Sakaguchi Y, Fujii N, Shoji T, et al. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One. 2014;9:e116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 29. Iguchi A, Watanabe Y, Iino N, Kazama JJ, Iesato H, Narita I. Serum magnesium concentration is inversely associated with fibroblast growth factor 23 in haemodialysis patients. Nephrology. 2014;19:667–671. [DOI] [PubMed] [Google Scholar]
- 30. Matsuzaki H, Kajita Y, Miwa M. Magnesium deficiency increases serum fibroblast growth factor‐23 levels in rats. Magnes Res. 2013;26:18–23. [DOI] [PubMed] [Google Scholar]
- 31. Matsuzaki H, Katsumata S, Maeda Y, Kajita Y. Changes in circulating levels of fibroblast growth factor 23 induced by short‐term dietary magnesium deficiency in rats. Magnes Res. 2016;29:48–54. [DOI] [PubMed] [Google Scholar]
- 32. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53:561–571. [DOI] [PubMed] [Google Scholar]
- 33. de Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int. 2009;75:677–681. [DOI] [PubMed] [Google Scholar]
- 34. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnes BA, Mendelson J. The measurement of exchangeable magnesium in dogs. Metabolism. 1963;12:184–193. [PubMed] [Google Scholar]
- 36. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. [DOI] [PubMed] [Google Scholar]
- 37. Bateman S. Disorders of magnesium: magnesium deficit and excess In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. St. Louis, MI: Elsevier Saunders; 2012:212–229. [Google Scholar]
- 38. Navarro‐Gonzalez JF, Mora‐Fernandez C, Garcia‐Perez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22:37–44. [DOI] [PubMed] [Google Scholar]
- 39. Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: the Honolulu heart study. Am J Clin Nutr. 1987;45:469–475. [DOI] [PubMed] [Google Scholar]
- 40. Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927–940. [DOI] [PubMed] [Google Scholar]
- 41. Resnick LM, Bardicef O, Altura BT, Alderman MH, Altura BM. Serum ionized magnesium: relation to blood pressure and racial factors. Am J Hypertens. 1997;10:1420–1424. [DOI] [PubMed] [Google Scholar]
- 42. Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta‐analysis. Eur J Clin Nutr. 2012;66:411–418. [DOI] [PubMed] [Google Scholar]
- 43. Joosten MM, Gansevoort RT, Mukamal KJ, et al. Urinary magnesium excretion and risk of hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension. 2013;61:1161–1167. [DOI] [PubMed] [Google Scholar]
- 44. Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. 2012;5:i25–i38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Touyz RM, Milne FJ, Reinach SG. Intracellular Mg2+, Ca2+, Na2+ and K+ in platelets and erythrocytes of essential hypertension patients: relation to blood pressure. Clin Exp Hypertens A. 1992;14:1189–1209. [DOI] [PubMed] [Google Scholar]
- 46. Kisters K, Krefting ER, Spieker C, Zidek W, Barenbrock M, Rahn KH. Increased Na+ and decreased Mg2+ intracellular concentrations in vascular smooth muscle cells from spontaneously hypertensive rats. Clin Sci. 1998;95:583–587. [DOI] [PubMed] [Google Scholar]
- 47. Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Vascular biology of magnesium and its transporters in hypertension. Magnes Res. 2010;23:S207–S215. [DOI] [PubMed] [Google Scholar]
- 48. Cunha AR, Medeiros F, Umbelino B, Oigman W, Touyz RM, Neves MF. Altered vascular structure and wave reflection in hypertensive women with low magnesium levels. J Am Soc Hypertens. 2013;7:344–352. [DOI] [PubMed] [Google Scholar]
- 49. Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end‐stage renal disease. Kidney Int. 1987;32:388–394. [DOI] [PubMed] [Google Scholar]
- 50. Cheng PT, Grabher JJ, LeGeros RZ. Effects of magnesium on calcium phosphate formation. Magnesium. 1988;7:123–132. [PubMed] [Google Scholar]
- 51. Pearson PJ, Evora PR, Seccombe JF, Schaff HV. Hypomagnesemia inhibits nitric oxide release from coronary endothelium: protective role of magnesium infusion after cardiac operations. Ann Thorac Surg. 1998;65:967–972. [DOI] [PubMed] [Google Scholar]
- 52. Satake K, Lee JD, Shimizu H, et al. Effects of magnesium on prostacyclin synthesis and intracellular free calcium concentration in vascular cells. Magnes Res. 2004;17:20–27. [PubMed] [Google Scholar]
- 53. Salem S, Bruck H, Bahlmann FH, et al. Relationship between Magnesium and Clinical Biomarkers on Inhibition of Vascular Calcification. Am J Nephrol. 2012;35:31–39. [DOI] [PubMed] [Google Scholar]
- 54. Ishimura E, Okuno S, Kitatani K, et al. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. [DOI] [PubMed] [Google Scholar]
- 55. Nakayama T, Nakayama H, Miyamoto M, Hamlin RL. Hemodynamic and electrocardiographic effects of magnesium sulfate in healthy dogs. J Vet Intern Med. 1999;13:485–490. [DOI] [PubMed] [Google Scholar]
- 56. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med. 2014;28:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jensen J, Henik RA, Brownfield M, Armstrong J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res. 1997;58:535–540. [PubMed] [Google Scholar]
- 58. Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin‐converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1995;76:1259–1265. [DOI] [PubMed] [Google Scholar]
- 59. Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM. Downregulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone‐infused mice: effects of magnesium. Hypertension. 2008;51:915–921. [DOI] [PubMed] [Google Scholar]
- 60. Ichihara A, Suzuki H, Saruta T. Effects of magnesium on the renin‐angiotensin‐aldosterone system in human subjects. J Lab Clin Med. 1993;122:432–440. [PubMed] [Google Scholar]
- 61. Atarashi K, Matsuoka H, Takagi M, Sugimoto T. Magnesium ion: a possible physiological regulator of aldosterone production. Life Sci. 1989;44:1483–1489. [DOI] [PubMed] [Google Scholar]
- 62. van Angelen AA, San‐Cristobal P, Pulskens WP, Hoenderop JG, Bindels RJ. The impact of dietary magnesium restriction on magnesiotropic and calciotropic genes. Nephrol Dial Transplant. 2013;28:2983–2993. [DOI] [PubMed] [Google Scholar]
- 63. Covic A, Passlick‐Deetjen J, Kroczak M, et al. A comparison of calcium acetate/magnesium carbonate and sevelamer‐hydrochloride effects on fibroblast growth factor‐23 and bone markers: post hoc evaluation from a controlled, randomized study. Nephrol Dial Transplant. 2013;28:2383–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bressendorff I, Hansen D, Schou M, et al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity—a prospective randomized double‐blinded placebo‐controlled clinical trial. Kidney Int Rep. 2017;2:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2‐SGK1 signaling pathway. Bone. 2012;51:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shimada T, Urakawa I, Yamazaki Y, et al. FGF‐23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. [DOI] [PubMed] [Google Scholar]
- 67. Andrukhova O, Smorodchenko A, Egerbacher M, et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2014;33:229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang B, Umbach AT, Chen H, et al. Up‐regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun. 2016;470:384–390. [DOI] [PubMed] [Google Scholar]
- 70. Toll J, Erb H, Bimbaum N, Schermerhorn T. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J Vet Intern Med. 2002;16:217–221. [DOI] [PubMed] [Google Scholar]
- 71. Diaz‐Tocados JM, Peralta‐Ramirez A, Rodriguez‐Ortiz ME, et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017;92:1084–1099. [DOI] [PubMed] [Google Scholar]
- 72. Barber PJ. Parathyroid gland function in the ageing cat [PhD thesis]. Royal Veterinary College. London: University of London; 1998:289. [Google Scholar]
- 73. McLeland SM, Lunn KF, Duncan CG, Refsal KR, Quimby JM. Relationship among serum creatinine, serum gastrin, calcium‐phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med. 2014;28:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sakaguchi Y, Shoji T, Hayashi T, et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end‐stage renal disease. Diabetes Care. 2012;35:1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sakaguchi Y, Tsubakihara Y, Rakugi H, Isaka Y. Does hypomagnesemia predict faster progression of nondiabetic chronic kidney disease? Am J Med. 2014;127:e13. [DOI] [PubMed] [Google Scholar]
- 76. Van Laecke S, Nagler EV, Vanholder R. The Reply. Am J Med. 2014;127:e15. [DOI] [PubMed] [Google Scholar]
- 77. Ross LA, Finco DR, Crowell WA. Effect of dietary phosphorus restriction on the kidneys of cats with reduced renal mass. Am J Vet Res. 1982;43:1023–1026. [PubMed] [Google Scholar]
- 78. Chakrabarti S, Syme HM, Brown CA, Elliott J. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol. 2013;50:147–155. [DOI] [PubMed] [Google Scholar]
- 79. Lawson J, Elliott J, Wheeler‐Jones C, Syme H, Jepson R. Renal fibrosis in feline chronic kidney disease: known mediators and mechanisms of injury. Vet J. 2015;203:18–26. [DOI] [PubMed] [Google Scholar]
- 80. Wooldridge JD, Gregory CR. Ionized and total serum magnesium concentrations in feline renal transplant recipients. Vet Surg. 1999;28:31–37. [DOI] [PubMed] [Google Scholar]
- 81. Norris CR, Nelson RW, Christopher MM. Serum total and ionized magnesium concentrations and urinary fractional excretion of magnesium in cats with diabetes mellitus and diabetic ketoacidosis. J Am Vet Med Assoc. 1999;215:1455–1459. [PubMed] [Google Scholar]
- 82. Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23:S194–S198. [DOI] [PubMed] [Google Scholar]


