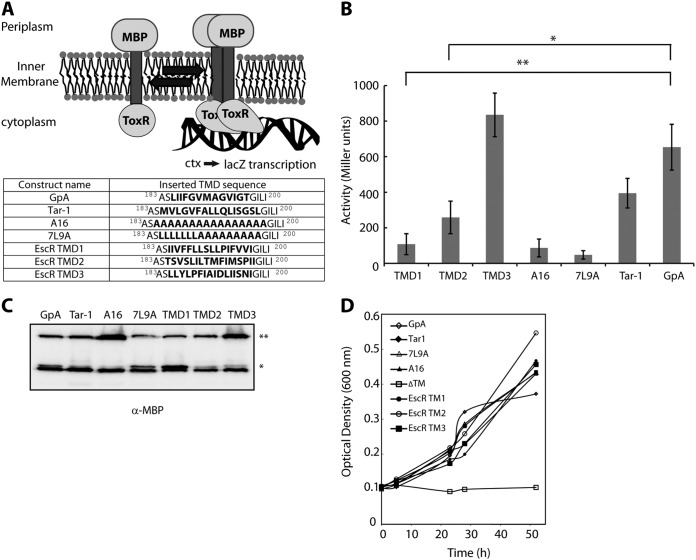
FIG 3 .
TMD3 of EscR promotes TMD self-oligomerization. (A) Schematic illustration of a ToxR assembly system. Oligomerization of the TMDs promotes the activation of the transcription activator ToxR, which binds the ctx promoter and initiates lacZ transcription. The TMD sequences that were inserted between the ToxR transcription activator and the maltose binding protein in the ToxR-TMD-MBP plasmid are presented. (B) FHK12 bacteria expressing a ToxR-TMD-MBP chimera were examined for LacZ activity. The activities of well-characterized dimerizing (GpA and Tar-1) and nondimerizing (A16 and 7L9A) TMDs are also shown. The oligomerization ability of the EscR TMD1 was low, TMD2 had moderate ability, and TMD3 showed very strong TMD oligomerization activity. Bars represent the average (+standard deviation) from at least three independent experiments. Statistical significance was determined by Student’s t test (**, P < 0.005; *, P < 0.01). (C) Samples of FHK12 cells containing the ToxR-TMD-MBP chimera proteins with different TMD sequences were lysed, separated, and immunoblotted using an anti-MBP antibody. The ToxR-TMD-MBP chimera protein (65 kDa) is marked with **, and the endogenous MBP (40 kDa) is marked with *. (D) Correct integration of the ToxR-TMD-MBP chimera proteins was tested by assessing their ability to functionally complement the malE deficiency of the PD28 bacterial strain. PD28 bacteria were transformed with plasmids expressing chimera proteins containing the EscR TMD1, TMD2, TMD3, GpA, Tar-1, 7L9A, or A16 or in the absence of a TMD (ΔTM) and were grown in a minimal medium containing maltose. As expected, the ΔTM negative control showed no growth, while all other constructs showed growth, indicating proper membrane integration.