Gurgel-Giannetti et al. describe a novel syndrome characterised by optic atrophy, sensory-motor neuropathy, recurrent rhabdomyolysis and reversible leukoencephalopathy, in two Brazilian families. Homozygosity mapping and exome sequencing reveal the cause to be a homozygous mutation in FDX2, which encodes a mitochondrial ferredoxin essential for iron–sulphur cluster biogenesis.
Keywords: FDX2, brain, nerve, muscle, optic atrophy
Abstract
Defects in iron–sulphur [Fe-S] cluster biogenesis are increasingly recognized as causing neurological disease. Mutations in a number of genes that encode proteins involved in mitochondrial [Fe-S] protein assembly lead to complex neurological phenotypes. One class of proteins essential in the early cluster assembly are ferredoxins. FDX2 is ubiquitously expressed and is essential in the de novo formation of [2Fe-2S] clusters in humans. We describe and genetically define a novel complex neurological syndrome identified in two Brazilian families, with a novel homozygous mutation in FDX2. Patients were clinically evaluated, underwent MRI, nerve conduction studies, EMG and muscle biopsy. To define the genetic aetiology, a combination of homozygosity mapping and whole exome sequencing was performed. We identified six patients from two apparently unrelated families with autosomal recessive inheritance of a complex neurological phenotype involving optic atrophy and nystagmus developing by age 3, followed by myopathy and recurrent episodes of cramps, myalgia and muscle weakness in the first or second decade of life. Sensory-motor axonal neuropathy led to progressive distal weakness. MRI disclosed a reversible or partially reversible leukoencephalopathy. Muscle biopsy demonstrated an unusual pattern of regional succinate dehydrogenase and cytochrome c oxidase deficiency with iron accumulation. The phenotype was mapped in both families to the same homozygous missense mutation in FDX2 (c.431C > T, p.P144L). The deleterious effect of the mutation was validated by real-time reverse transcription polymerase chain reaction and western blot analysis, which demonstrated normal expression of FDX2 mRNA but severely reduced expression of FDX2 protein in muscle tissue. This study describes a novel complex neurological phenotype with unusual MRI and muscle biopsy features, conclusively mapped to a mutation in FDX2, which encodes a ubiquitously expressed mitochondrial ferredoxin essential for early [Fe-S] cluster biogenesis.
Introduction
Iron–sulphur [Fe-S] clusters are essential biological cofactors found in all living organisms, from archaea to eukaryotes (Fontecave, 2006; Lill, 2009). They consist of protein-bound Fe ions, linked by sulphide bridges and are essential in a large number of electron transfer reactions. [Fe-S] clusters are almost unique in their ability to access various redox states over a wide range, with electron transport roles in critical processes like the mitochondrial respiratory chain. [Fe-S] clusters have also been found to play important roles in more diverse cellular pathways from catalysis to gene regulation (Beinert et al., 1997; Rouault, 2015). The biogenesis of [Fe/S] proteins in the mitochondria is a complex process and takes place in several stages (Braymer and Lill, 2017). The early stage of biogenesis involves a cysteine desulphurase as a source of sulphur and frataxin as a potential source of iron, with the cluster being formed on the scaffold protein iron-sulphur cluster assembly enzyme (ISCU). The newly made [2Fe-2S] cluster is then transferred to numerous downstream biogenesis proteins that will use the cofactor for transfer to target [Fe/S] proteins such as the subunits of mitochondrial complexes I and II.
Another class of proteins essential for de novo [2Fe-2S] cluster assembly on ISCU are ferredoxins (Webert et al., 2014; Boniecki et al., 2017). Humans possess two ferredoxins, FDX1 and FDX2 (formerly FDX1L) (Sheftel et al., 2010). While both proteins localize to the mitochondrion, expression of FDX1 is highest in the adrenal gland, where the protein functions in the steroid synthesis pathway. FDX2, however, is ubiquitously expressed and is essential in the formation of [Fe-S] clusters in humans (Sheftel et al., 2010).
Mutations in a number of genes that encode proteins involved in [Fe-S] cluster assembly lead to complex neurological phenotypes (Stehling et al., 2014). The most common of these syndromes is Friedreich’s ataxia, an autosomal recessive multisystem neurodegenerative disorder characterized by ataxia, peripheral neuropathy and cardiomyopathy (Beilschmidt and Puccio, 2014). It is typically caused by a trinucleotide repeat intronic expansion in FXN, encoding frataxin (Dürr et al., 1996). Loss of function mutations in ISCU lead to myopathy with severe exercise intolerance (Mochel et al., 2008; Olsson et al., 2008; Nordin et al., 2011; Legati et al., 2017) and mutations in NUBPL, an [Fe-S] protein involved in complex I assembly (Sheftel et al., 2009), lead to leukoencephalopathy, ataxia and developmental regression (Calvo et al., 2010). In 2014, a single case was described with isolated mitochondrial myopathy due to a homozygous translation start site mutation in FDX2 (Spiegel et al., 2014). More recently, mutations in FDXR, encoding ferredoxin reductase, were described in eight patients with a syndrome including hearing loss, optic atrophy and sensory neuropathy (Paul et al., 2017).
In this study, we describe the clinical phenotype of six patients from two apparently unrelated Brazilian families but living in the same geographic region with a complex phenotype including optic atrophy, reversible leukoencephalopathy, mitochondrial myopathy with exercise intolerance and axonal polyneuropathy and, in some patients, pyramidal signs. Using a combination of homozygosity mapping and whole exome sequencing, we mapped this phenotype to the same homozygous, deleterious mutation in FDX2.
Materials and methods
Subjects
Clinical evaluation was performed on both families by two independent research groups that shared their data. All patients were clinically and neurologically evaluated, and underwent EMG and nerve conduction studies and muscle biopsy.
We received approval of the ethical standards committee at both institutions (São Paulo University and Federal University of Minas Gerais) and written informed consent was obtained from all included family members.
Neuroimaging
All patients underwent at least one exam. We analysed 13 brain MRIs performed at different ages ranging from 8 months to 41 years. Since MRIs were performed in different centres, analysis was based mainly on multiplanar FLAIR, T1 and T2-weighted images. Intensity of T2 changes were graded in a semi-quantitative manner as absent, mild, moderate or severe. Diffusion-weighted images and apparent diffusion coefficient maps, when available, were scrutinized in the search for lesions with restricted diffusion. Magnetic resonance spectroscopy was also analysed when available, and we focused on the detection of lactate peaks.
Muscle biopsy
Muscle biopsies were performed on the biceps brachialis and cryostat sections were obtained with 8 µm thickness. Conventional histochemical techniques were performed: haematoxylin and eosin, modified Gomori trichrome, Periodic acid–Schiff technique, Oil Red O, reduced nicotinamide adenine dinucleotide dehydrogenase-tetrazolium reductase (NADH-TR), succinate dehydrogenase (SDH), cytochrome c oxidase (COX), and adenosine triphosphatase (ATPase) preincubated at pH 9.4, 4.63 and 4.35. Perls’ Prussian Blue staining was performed as described (Stevens, 1990) and with DAB enhancement (Mochel, 2008) to identify iron deposition.
For immunohistochemistry, 8 μm sections were fixed in 4% formaldehyde at 4°C for 10 min, washed in Tris-buffered saline-Tween 20 (TBS-T) for 10 min, permeabilized in a graded methanol series (70% 10 min, 95% 10 min, 100% 20 min, 95% 10 min, 70% 10 min), washed in TBS-T for 5 min and further processed in a Dako Autostainer using the Dako EnVision™ FLEX High pH kit. The following antibodies were applied for 1 h: anti-SDHB ab14714 Abcam (1:500), anti-MTCO1 ab14705 Abcam (1:2000) and anti-VDAC1 ab14734 Abcam (1:2000).
Electron microscopy was performed on tissue specimens that had been fixed in glutaraldehyde, post-fixed in OsO4 and embedded in resin. Ultrathin sections were contrasted with uranyl acetate and lead citrate.
The respiratory chain enzyme analysis was performed on frozen tissue using spectrophotometry (DiMauro et al., 1987).
Molecular analysis
Genome wide genotyping was performed on Patients 1–4, both parents and an unaffected sibling from Family 1 using the Axiom® Genome-Wide LAT 1 Array (Affymetrix). Runs of homozygosity were identified using PLINK software (Purcell et al., 2007). Whole exome sequencing was performed on Patients 3 and 4 from Family 1 and in four members (Patients 5, 6 and parents of Patient 5) from Family 2 using the Agilent SureSelect Exon Enrichment kit. Segregation of the FDX2 mutation was confirmed by Sanger sequencing of FDX2 exon 5 using the following primer sequences: Forward: 5′-ATCCTCCCCACTTCCAGTTC-3′; Reverse: 5′-TCTTAAGCTCCTGGCCTCAA-3′.
Prior to whole exome sequencing, Sanger sequencing of EARS2, SDHA and SDHB was performed in Patient 5, and no potential pathogenic variant was identified in homozygosity or compound heterozygosity in this subject. Cytogenetic investigation was performed on cultured peripheral blood lymphocytes from Patient 1 and her parents, including G- and C-banding, and fluorescent in situ hybridization (FISH) analyses.
Functional studies
Human RNA extraction, cDNA synthesis and RT-qPCR
RNA extracted from muscle from four patients and two healthy controls was used for quantitative reverse transcription PCR (RT-qPCR). RNA extraction was performed using TRIzol® (ThermoFisher Scientific) and RNA quality was evaluated by agarose gel electrophoresis; and all samples had 260:230 ratio above 1.90, measured using a NanoDrop (ThermoFisher Scientific). Total RNA (1 µg/µl) was reverse-transcribed with oligo(dT) primers using SuperScript™ III First-strand Synthesis System (ThermoFisher Scientific). FDX2 primers for RT-qPCR spanned the exon 4–5 junction: Forward 5′- GTGAGTGAAGACCACCTGGAT-3′; Reverse 5′- GCCATGTCTAGCATGTCGTC-3′.
RT-qPCR was normalized to TBP and the cDNA amplification was performed using Applied Biosystems® 7500 Fast Real-time PCR System. Relative gene expression was calculated using 2−ΔΔCT method (Schmittgen and Livak, 2008). Each experiment was performed in triplicate considering standard deviation of triplicate positive when <0.1.
Western blot study
Muscle tissue was lysed in RIPA buffer (Pierce) and protein concentration was determined with Pierce 660 nm protein assay. Lysed skeletal muscle samples were subjected to SDS-PAGE (6–18% gradient gel; Stehling et al., 2018) and immunostaining. Antibodies were targeted against FDX2 (affinity purified, Sheftel et al., 2010), F1 α/β subunits of complex V (kind gift of Drs H. Schägger and I. Wittig, 1:1000), VDAC (Cell Signalling Technology, 1:1000), tubulin (Sigma, 1:10 000), and actin (BD Transduction Laboratories, 1:2000).
Results
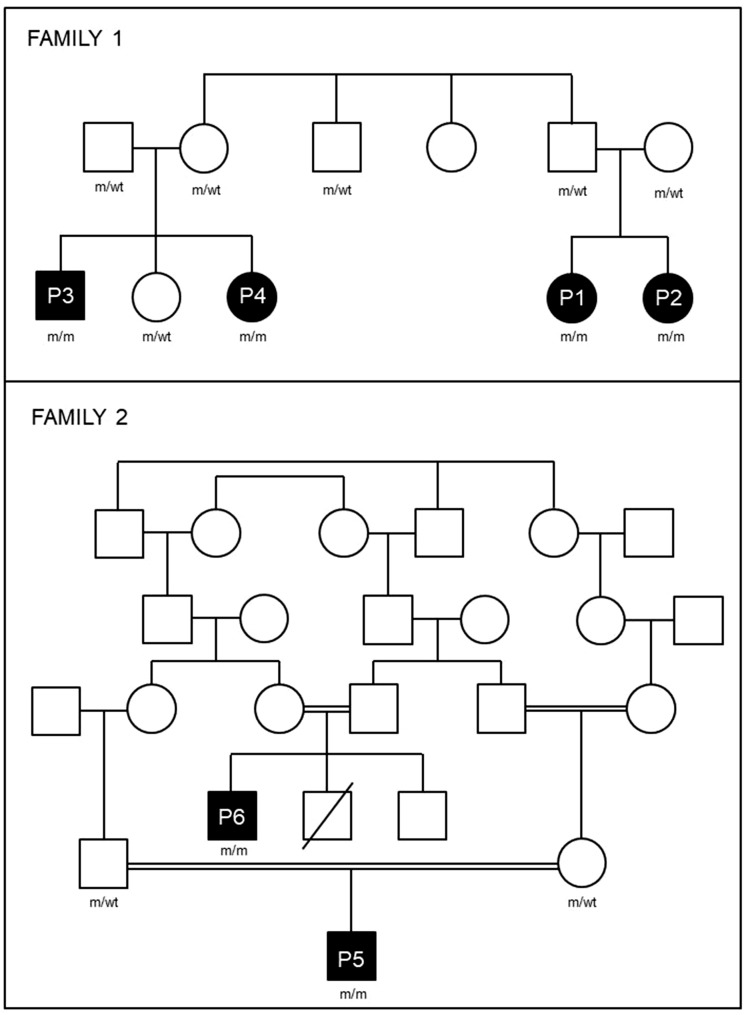
We identified six patients from two apparently unrelated Brazilian families living in the same geographic area with a complex multisystem phenotype: childhood onset of optic atrophy, recurrent episodes of myalgia and/or proximal weakness, axonal polyneuropathy, pyramidal signs (present in two patients) and reversible or partially reversible leukoencephalopathy on MRI. A summary of clinical findings is presented in Tables 1 and 2, and a simplified pedigree of Families 1 and 2 is shown in Fig. 1.
Table 3.
Summary of the neuroimaging findings from the six patients performed at different ages
| Age | Case/MR number | Subcortical white matter (T2 hypertensity) | Thalamus (T2 hypertensity) | Brainstem (T2 hypertensity) | DWI hyperintensity | Restricted diffusion (ADC map) | Corpus callosum (thinning) | MRS (lactate peaks) |
|---|---|---|---|---|---|---|---|---|
| 8 m | P1/01 | + | – | – | NA | NA | Mild global; posterior predominance | NA |
| 1 y 8 m | P2/01 | ++ | + | + | Yes | Yes | Isthmus | NA |
| 3 y | P2/02 | +++ | +++ | +++ | Yes | No (facilitated diffusion) | Isthmus | NA |
| 3 y | P5/01 | +++ | +++ | +++ | Yes | NA | Isthmus | Yes |
| 5 y | P1/02 | +++ | ++ | ++ | Yes | NA | Mild global; posterior predominance | NA |
| 6 y | P5/02 | ++ | + | + | Yes | Yes | Isthmus | Yes |
| 7 y | P5/03 | + | + | + | Yes | Yes | Isthmus | NA |
| 9 y | P5/04 | – | – | – | No | No | Isthmus | No |
| 9 y | P1/03 | + | – | + | Yes | Yes | Mild global; posterior predominance | No |
| 10 y | P1/04 | – | – | + | No | No | Mild global; posterior predominance | No |
| Adult | P3/01 | + | – | – | No | No | Mild global; posterior predominance | No |
| Adult | P4/01 | – | – | – | No | No | Normal | NA |
| Adult | P6/01 | – | – | – | No | No | Normal | No |
ADC = apparent diffusion coefficient; DWI = diffusion-weighted image; MR = magnetic resonance; NA = not available. T2-hyperintensity changes were graded using a semiquantitative scale: absent (–); mild (+); moderate (++) and severe (+++).
Table 1.
Summary of clinical findings
| Patient | Current age | Age of onset | First symptoms | Developmental delay | Nystagmus/ optic atrophy | Exercise-induced myalgia/ myopathy | Axonal sensori-motor polyneuropathy | Endocrine abnormalities | Haematological abnormalities | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical hypothyroidism | Type II diabetes | Neutropaenia | Microcytic anaemia | ||||||||
| P1 | 13 y | <6 m | Floppy baby | Global | + | + (eo) | + | + | – | + | + |
| P2 | 6 y | 1 y 4 m | Nystagmus, LVA | Minor motora | + | + (eo) | _ | + | – | + | + |
| P3 | 29 y | 1 y | Nystagmus, LVA | Minor motora | + | + (to) | + | + | – | + | + |
| P4 | 34 y | 1 y | Nystagmus, LVA | Minor motora | + | + (to) | + | NA | + (ao) | + | + |
| P5 | 11 y | 8 m | Nystagmus, LVA | – | + | + (eo) | – | – | – | – | – |
| P6 | 41 y | 6 m | Nystagmus, LVA | Minor motora | + | + (eo) | + | – | + (+++ao) + | _ | _ |
aPatients walked at around 2 years of age. + = present; – = absent; ao = adult-onset; eo = early-onset; LVA = low visual acuity; NA = not available; to = teenage-onset.
Table 2.
Summary of the neurological findings
| Patient | Learning disability | Low visual acuity and optic atrophy | Ptosis | Proximal weakness | Distal weakness | Pes cavus | Sensory abnormalities | DTRs | Spasticity | Babinski sign |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | + | + | + | – | – | + | – | H | – | – |
| P2 | – | + | – | – | – | – | – | H | – | – |
| P3 | – | + | + | + | + | + | + | H | Mild | + |
| P4 | – | + | – | – | – | + | + | H | Mild | + |
| P5 | – | + | – | – | – | – | – | N | – | – |
| P6 | – | + | + | – | + | + | + | H | – | – |
+ = present; – = absent; DTRs = deep tendon reflexes; H = hypoactive; N = normoactive.
Figure 1.
Pedigree of Families 1 and 2. Filled boxes are affected members and unfilled boxes are unaffected. Genotypes are shown where m = mutant, wt = wild type.
Clinical findings
The first recognizable clinical sign in all patients was nystagmus, secondary to low visual acuity caused by non-progressive optic atrophy, seen before the age of 3 years in all patients. A mild motor delay was observed in all patients, except Patient 1, who had a global developmental delay, and Patient 5, who had a normal development.
In all patients there was evidence of recurrent episodes of cramps, myalgia and muscle weakness, often precipitated by exercise, infections or low temperature. These events started in childhood or adolescence, sometimes leading to temporary loss of ambulation for 1 to 3 weeks. In Patient 5, a creatine kinase level during an episode was >3000 U/l, indicating rhabdomyolysis.
Motor features included a combination of myopathy, upper motor neuron dysfunction and sensori-motor axonal neuropathy. Weakness was progressive in Patient 6, who became wheelchair-bound at the age of 33 years. Hypoactive deep tendon reflexes were seen in all individuals, except Patient 5. Babinski signs with mild spasticity were seen in Patients 3 and 4. Sensory ataxia, characterized by positive Romberg sign and decreased vibration and position sensitivity, was present in Patients 3 and 4. Permanent weakness was related to age and disease duration, since it was present in two of the three adult patients: Patient 3 had proximal and distal involvement, and Patient 6 only distal weakness. Clinical features of neuropathy and abnormal nerve conduction study were seen in all four older patients with more than 10 years of age. Finally, ptosis was seen in Patients 3 and 6 (Table 2).
In Family 1, all tested members (Patients 1–4) had mild microcytic anaemia without iron deficiency, sometimes accompanied by thrombocytopaenia or neutropaenia.
All patients underwent nerve conduction studies and EMG, which showed sensori-motor polyneuropathy in Patients 1, 3, 4 and 6, but no abnormalities in Patients 2 and 5.
Additionally, Patient 1 had global developmental delay, intellectual disability and dysmorphic features, characterized by protruding ears, micrognathia, high arched palate, almond-shaped eyes, high nasal bridge, dolicocephaly, and brachydactyly. The dysmorphic features were thought to be due to an unrelated and separate condition not affecting the other five patients described here.
Neuroimaging
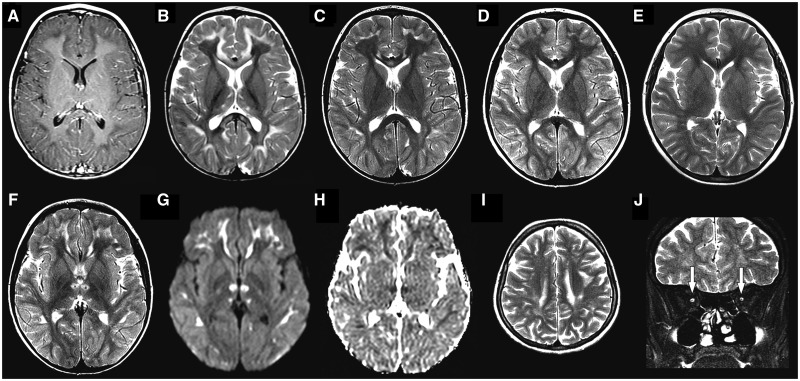
The common finding observed in brain MRI from all patients was the presence of hypoplastic optic nerves and chiasm (Fig. 2). In three patients, magnetic resonance abnormalities progressed in a somewhat similar fashion (Patients 1, 2 and 5). In Patient 2 the first exam done at 1 year and 8 months demonstrated T2 hyperintensity involving subcortical and deep cerebral white matter and corpus callosum (genu and splenium), associated with restricted diffusion. There was also mild pontine T2 hyperintensity sparing both corticospinal tracts and some thinning of the isthmus of the corpus callosum. Magnetic resonance findings were more evident between 3 and 5 years of age, not only the white matter lesions, but also the pontine changes and a peculiar pattern of thalamic involvement. This pattern was characterized by T2 hyperintensity and restricted diffusion in anterior nuclei, sparing mammillothalamic tracts and extending also to the internal medullary lamina.
Figure 2.
Neuroimaging findings. In Subject 5, at age 3 years, axial T1-weighted post-contrast image (A) demonstrates hypointensity in the subcortical white matter, without abnormal enhancement. Axial T2-weighted images from the same patient at ages 3 (B), 6 (C), 7 (D) and 9 years (E) disclose progressive improvement of T2 hyperintense abnormalities in thalami, subcortical white matter and corpus callosum, initially appreciated in B. Axial T2-weighted image from the same patient at age 6 years (F) shows sparing of mammillothalamic tracts, seen as T2 hypointense dots in the centre of the signal change in the anterior portion of thalami. Considering the subsequent improvement, the restricted diffusion [hyperintensity in diffusion image (G) and hypointensity in apparent diffusion coefficients map (H)] probably reflects intramyelinic oedema. Axial and coronal T2-weighted images from Subject 3 at age 27 years (I and J), demonstrate residual T2 hyperintensity in subcortical white matter (I) and hypoplasia of both optic nerves (arrows in J).
By the age of 7 to 9 years of life, MRI changes presented remarkable improvement. In two patients, Patients 1 and 5, MRI exams at this age became essentially normal, except for some callosal thinning. In two patients (Patients 4 and 6), brain imaging was obtained at adult life and the exams were otherwise normal (except for the optic pathway involvement). In the youngest adult patient (Patient 3) there were also patchy T2 hyperintense abnormalities throughout the supratentorial white matter (without diffusion changes) and reduced thickness of the corpus callosum, particularly the posterior portions.
Lactate peaks were found using magnetic resonance spectroscopy in only one patient (Patient 5), from the four patients who had at least one magnetic resonance spectroscopy exam in our series. In this patient lactate peaks were found when the patient presented significant structural MRI changes (at 3 and 6 years old), and were not present when structural MRI was essentially normal (at age 9). In the other three patients the magnetic resonance spectroscopy was performed when the brain MRI has mild white matter abnormalities (Patients 1 and 3) or when it was normal (Patient 5).
In Patients 1, 2 and 5, who were clinically evaluated at the time that the brain magnetic resonance showed the white matter, thalamus and brainstem abnormalities, there was no evidence of any pyramidal or extrapyramidal signs in the neurological examination. Only one patient was submitted to an MRI during the first year of life, at the age of 8 months, and this exam revealed some widening of the lateral ventricles and mild diffuse T2 hyperintensity in white matter (suggesting at least some delay in myelination) (Patient 1). However, this patient has also a coincidental chromosomal abnormality.
Muscle pathology
Patients 3–6 underwent muscle biopsy and in all samples there was the presence of ragged red fibres and SDH and COX negative fibres. Respiratory chain enzyme analysis was only performed in Patient 5 and showed no abnormalities. In Patient 5, the muscle biopsy was performed at 3 years of age and the proportion of ragged red fibres was less frequent than in the others patients. The other three patients (Patients 3, 4 and 6) underwent muscle biopsy in adult life.
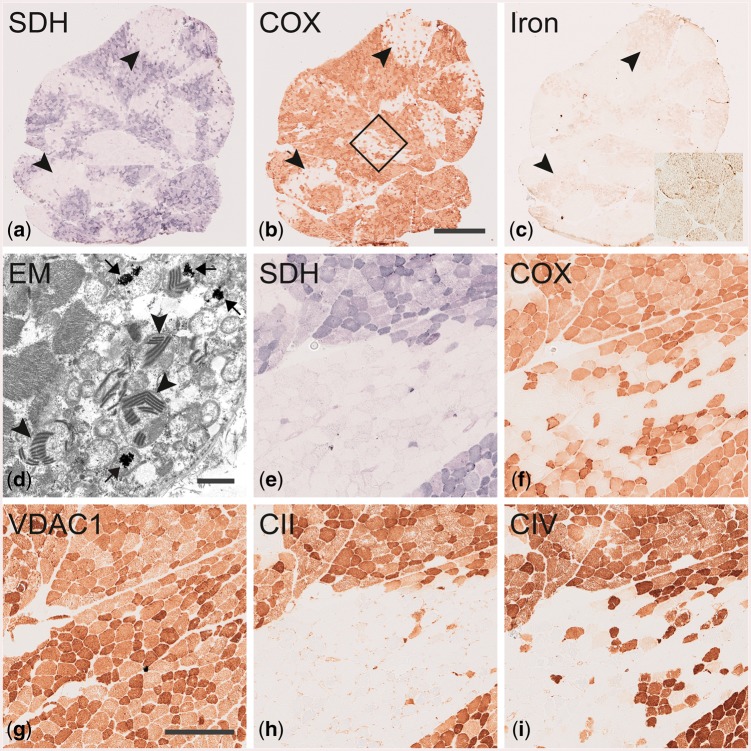
In Patient 3, the muscle showed more intense alterations, which are illustrated in Fig. 3.
Figure 3.
Muscle biopsy from Patient 3 demonstrating mitochondrial myopathy and iron deposition. (A–C) are serial sections showing an overview of the entire muscle biopsy cross-section. Note the focal deficiency of SDH and COX affecting numerous adjacent fibres in A and B, respectively and the associated deposition of iron in C. Inset in C shows fibres at higher magnification and punctate staining of iron. Electron microscopy in D shows electron dense inclusions presumably containing iron in mitochondria (arrows) and paracrystalline inclusions in mitochondria (arrowheads). (E–I) Serial sections demonstrating deficiency of complex II (H; subunit SDHB) and complex IV (I; subunit CO1) corresponding to the focal enzyme histochemical deficiency of SDH (E) and COX (F). Mitochondria are present in the corresponding regions as revealed by VDAC1 immunohistochemistry (G). The box in B marks the region shown at higher magnification in serial sections (E–I). Scale bar = 1 mm in A–C (bar shown in B). Scale bar in D = 1 µm. Scale bar = 250 µm in E–I (bar shown in G).
There were SDH- and COX-negative fibres that were clustered together in patch-like areas (Fig. 3A and B). Furthermore, punctate iron accumulation was observed in SDH and COX deficient fibres by iron staining (Fig. 3C). Electron microscopy revealed mitochondrial paracrystalline inclusions in numerous fibres. In these fibres frequent electron-dense irregular inclusions were identified that presumably represent iron deposition in the mitochondria (Fig. 3D). Immunohistochemical analysis of complex II and complex IV subunits showed markedly reduced expression in fibres with low SDH and COX enzymatic activity (Fig. 3E, F, H and I) while mitochondrial mass was increased as revealed by the mitochondrial marker VDAC1 (porin; Fig. 3G).
Genetic results
Genome-wide homozygosity mapping in all affected members of Family 1 revealed two shared homozygous regions, a 1 Mb region on Chromosome 19 (Chr19: 9400194–10492040) and a 500 kb region on Chromosome 2 (Chr2: 89469319–89994709). We next examined the whole exome sequencing data from Family 1, limiting our analysis to rare, homozygous variants that were not synonymous or intronic and mapped within the regions of homozygosity identified. The only such variant detected was in FDX2 (NM_001031734 c.431C > T, p.P144L). This variant was not found in the ExAC or 1000G databases but was found in the heterozygous state in 1 of 600 controls from the Brazilian ABraOM database (minor allele frequency: 0.000821) (Naslavsky et al., 2017). We validated the variant by Sanger sequencing, confirming that all affected family members were homozygous, whereas the patients’ parents and an unaffected sibling were all heterozygous. Patient 1, who had some additional dysmorphic features not seen in the other five patients described, was found to carry a coincidental tetrasomy for the short arm of chromosome 9, karyotype: 46,XX,+i(9)(p10). Both her parents had normal karyotypes.
Family 2 underwent independent genetic studies. The index case (Patient 5) was first investigated for mutations in EARS2 (Steeweg et al., 2012), because of the reversible leukoencephalopathy, and for mutations in the SDHA and SDHB (Alston et al., 2012), based on the presence of SDH negative fibres in muscle biopsy. No mutations were identified in these genes. Whole exome sequencing analysis was performed in four members of this family and the same FDX2 mutation detected in Family 1 was identified as the only segregating variant, homozygous in the two affected patients (Patients 5 and 6) and heterozygous in the parents of Patient 5.
The FDX2 p.P144L variant affects a highly conserved residue that is identical in all mammals with an orthologous gene, and in invertebrates such as Drosophila melanogaster and Caenorhabditis elegans.
Functional validation of the FDX2 mutation
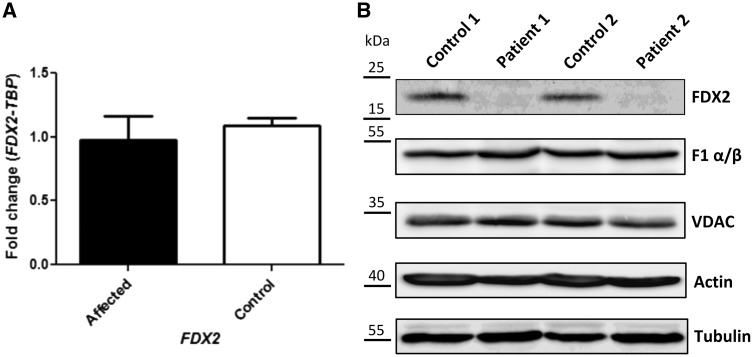
Real-time RT-PCR was performed using muscle samples from Patients 3–6 and control muscle from two unrelated individuals. We identified no difference in FDX2 mRNA expression between patients and controls (Fig. 4A).
Figure 4.
Functional studies. (A) RT-qPCR showing relative FDX2 expression normalized to TBP in patients and controls. (B) Western blotting in patient and control muscle. Immunostaining was performed using specific antibodies against the mitochondrial protein FDX2, the F1 α/β subunits of complex V, and the voltage-dependent anion channel (VDAC, porin). Staining against actin and tubulin served as a loading control.
To determine whether the mutation affected protein stability, we examined the FDX2 protein levels in muscle from Patients 3 and 6 (one patient from each family) and two unrelated controls by immunostaining. This revealed a severe reduction of FDX2 levels in patients as compared to controls. Equal protein loading was confirmed by Ponceau S staining (not shown) and by immunoblotting for a number of other mitochondrial (F1 α/β, VDAC) and cytosolic (actin, tubulin) proteins (Fig. 4B). The low amount of FDX2 in patient samples provides additional evidence that the FDX2 p.P144L mutation is pathogenic.
Discussion
In this study, we describe a novel childhood onset disorder in two families with unusual clinical, radiological and pathological features. The clinical syndrome included early-onset optic atrophy, recurrent episodes of myalgia with progressive myopathy, axonal polyneuropathy and a variety of haematological and endocrine abnormalities including microcytic anaemia, subclinical hypothyroidism, diabetes mellitus and reversible leukoencephalopathy. We used a combination of homozygosity and whole exome sequencing to identify the cause as a recurrent homozygous mutation in FDX2. We validated our findings genetically through segregation analysis and biochemically by demonstrating the almost complete absence of FDX2 protein by western blot in affected patient tissue.
Our clinical assessments of six patients with ages ranging from childhood to adult life allow us to infer the natural history of this disorder. Nystagmus and optic atrophy was the first sign of the disease and was present in the first 3 years of life in all six patients. Myopathic features developed during the first or second decade of life and presented with mild proximal weakness in limbs and/or recurrent acute episodes of myalgia and weakness precipitated by triggers such as cold weather and infections. Axonal neuropathy developed in the second decade or in adulthood leading to progressive distal weakness.
Interestingly, two individual reports have implicated mutations in FDX2 causing a relatively discrete phenotype of mitochondrial myopathy and rhabdomyolysis without the additional features seen in our patients, or the unique radiological or pathological changes described here (Spiegel et al., 2014; Lebigot et al., 2017). Both previously reported cases shared the same mutation affecting the ATG start codon of FDX2 (c.1A > T) and was predicted to lead to loss of FDX2 expression. Despite normal mRNA levels of FDX2, we could demonstrate severely reduced protein expression in patient tissue, which could represent instability or excess degradation of misfolded or otherwise dysfunctional protein.
The extensive and multisystem involvement in our patients is reflected by the phenotype associated with mutations in the gene of an essential FDX2 interaction partner within the [Fe-S] cluster assembly machinery, FDXR, encoding ferredoxin reductase. These patients develop optic atrophy in addition to deafness and sensory neuropathy (Paul et al., 2017).
Leukoencephalopathy is reported in several other disorders of [Fe-S] biogenesis, e.g. NFU1 and NUBPL mutations. In NUBPL mutations (Kevelam et al., 2013; Ahting et al., 2015), some patients show progressive improvement of signal abnormality in the corpus callosum and cerebral white matter, although this occurs in the setting of clinical worsening over time (Kevelam et al., 2013). In our patients we observed a progressive improvement of the white matter, thalamus and brainstem lesions compatible with reversible or partially reversible leukoencephalopathy. This finding is intriguing and further studies will be necessary to explain the improvement of the cerebral abnormalities. However defects in MLC1, HEPACAM and CLCN2 cause leukoencephalopathies characterized by intramyelinic oedema (T-hyperintensity in subcortical white matter and corpus callosum, associated with restricted diffusion), and in some the intramyelinic oedema can be reversible (Depienne et al., 2013). This may explain the reversibility seen in our patients in whom intramyelinic oedema was a feature.
Interestingly, we observed that the leukoencephalopathy did not show a clinical correlation. None of the three patients (Patients 1, 2 and 5) demonstrated any pyramidal or extrapyramidal signs at the time that the brain MRI demonstrated white matter, thalamus and brainstem abnormalities. Therefore, we can conclude the leukoencephalopathy related to the FDX2 p.P144L mutation was reversible and asymptomatic in the patients here described. However, in two adult patients mild upper motor neuron signs were observed indicating that the pyramidal tracts may become involved over time. Follow-up of these patients can clarify this neurological finding.
The muscle histology in our patients is unusual, with a pattern characterized by fibres with mitochondrial proliferation in Gomori trichrome staining, and clusters of fibres that were SDH- and COX-negative. This histochemical abnormality is unusual for classical mitochondrial myopathies in which the ragged red fibres are SDH positive and COX negative (DiMauro and Gurgel-Giannetti, 2005). The histopathological findings are also different to findings in most cases of mitochondrial complex II deficiency, in which there are ragged red fibres that are SDH negative but positive in the COX reaction (Alston et al., 2012). These findings are consistent with the function of FDX2 in the [Fe/S] cluster assembly of respiratory complexes I-III and heme A production for complex IV (Sheftel et al., 2010). The immunohistochemical deficiency of subunits of complexes II and IV showed the same distribution as the enzyme histochemical deficiency of COX and SDH indicating that defective assembly of the respiratory chain complexes cause the enzyme deficiency. The patchy distribution of deficiency and variability from one cell to another remains unexplained.
The peculiar and characteristic regional SDH and COX deficiency and iron accumulation as revealed by iron staining and electron microscopy observed here have also been reported in ISCU myopathy (Olsson et al., 2008; Kollberg et al., 2009). These similarities suggest that both ISCU and FDX2 are important for cellular iron homeostasis via their role in the maturation of cytosolic IRP1 to an aconitase (Paul and Lill, 2015).
Defects in [Fe-S] cluster biogenesis are increasingly recognized as causing neurological disease. They play a critical role in mitochondrial function, which is exemplified by the varied neurological features in these cases, affecting the CNS as well as peripheral nerve and muscle. In conclusion, this study reveals that a biallelic mutation in FDX2 causes a complex childhood onset syndrome with unusual histopathological features, and confirms the important role that FDX2 plays in mitochondrial health and disease.
Acknowledgements
Maurilio Pacheco e a Larissa Torres: logistic support in Alfenas (MG, Brazil).
Funding
This work was in part financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Fapemig, FAPESP-CEPID e INCT-CNPq; The Leonard Wolfson Experimental Neurology Centre, The Medical Research Council (MRC) and the Wellcome Trust. R.L. acknowledges generous financial support from Deutsche Forschungsgemeinschaft (SPP 1927) and networking support from the COST Action FeSBioNet (Contract CA15133). The Swedish Research Council Proj no 2012-02014 (to A.O.).
Glossary
Abbreviations
- [Fe-S]
iron–sulphur
- COX
cytochrome c oxidase
- SDH
succinate dehydrogenase
References
- Ahting U, Mayr JA, Vanlander AV, Hardy SA, Santra S, Makowski C et al. . Clinical, biochemical, and genetic spectrum of seven patients with NFU1 deficiency. Front Genet 2015; 6: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston CL, Davison JE, Meloni F, van der Westhuizen FH, He L, Hornig-Do HT et al. . Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Genet 2012; 49: 569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilschmidt LK, Puccio HM. Mammalian Fe-S cluster biogenesis and its implication in disease. Biochimie 2014; 100: 48–60. [DOI] [PubMed] [Google Scholar]
- Beinert H, Holm RH, Münck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 1997; 277: 653–9. [DOI] [PubMed] [Google Scholar]
- Boniecki MT, Freibert SA, Muhlenhoff U, Lill R, Cygler M. Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex. Nat Commun 2017; 8: 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braymer JJ, Lill R. Iron-sulfur cluster biogenesis and trafficking in mitochondria. J Biol Chem 2017; 292: 12754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Tucker EJ, Compton AG, Kirby DM, Crawford G, Burtt NP et al. . High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat Genet 2010; 42: 851–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Bugiani M, Dupuits C, Galanaud D, Touitou V, Postma N et al. . Brain white matter oedema due CIC-2 chloride chanel deficiency: an observational analytical study. Lancet Neurol 2013: 12: 659–68. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Gurgel-Giannetti J. The expanding phenotype of mitochondrial myopathy. Curr Opin Neurol 2005; 18: 538–42. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Servidei S, Zeviani M, DiRocco M, DeVivo DC, DiDonato S et al. . Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol 1987; 22: 498–506. [DOI] [PubMed] [Google Scholar]
- Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C et al. . Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med 1996; 335: 1169–75. [DOI] [PubMed] [Google Scholar]
- Fontecave M. Iron-sulfur clusters: ever-expanding roles. Nat Chem Biol 2006; 2: 171–4. [DOI] [PubMed] [Google Scholar]
- Kevelam SH, Rodenburg RJ, Wolf NI, Ferreira P, Lunsing RJ, Nijtmans LG et al. . NUBPL mutations in patiens with complex I deficiency and distinct MRI pattern. Neurology 2013; 80: 1577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollberg G, Tulinius M, Melberg A, Darin N, Andersen O, Holmgren D et al. . Clinical manifestation and a new ISCU mutation in iron–sulphur cluster deficiency myopathy. Brain 2009; 132(Pt 8): 2170–9. [DOI] [PubMed] [Google Scholar]
- Lebigot E, Gaignard P, Dorboz I, Slama A, Rio M, de Lonlay P et al. . Impact of mutations within the [Fe-S] cluster or the lipoic biosynthesis pathways on mitochondrial protein expression profiles in fibroblast from patients. Mol Genet Metab 2017; 122: 85–94. [DOI] [PubMed] [Google Scholar]
- Legati A, Reyes A, Ceccatelli Berti C, Stehling O, Marchet S, Lamperti C et al. . A novel de novo dominant mutation in ISCU associated with mitochondrial myopathy. J Med Genet 2017; 54: 815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R. Function and biogenesis of iron-sulphur proteins. Nature 2009; 460: 831–38. [DOI] [PubMed] [Google Scholar]
- Mochel F, Knight MA, Tong WH, Hernandez D, Ayyad K, Taivassalo T et al. . Splice mutation in iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am J Hum Genet 2008; 82: 652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky MS, Yamamoto GL, de Almeida TF, Ezquina SAM, Sunaga DY, Pho N et al. . Exomic variants of an elderly cohort of Brazilians in the ABraOM database. Hum Mutat 2017; 38: 751–63. [DOI] [PubMed] [Google Scholar]
- Nordin A, Larsson E, Thornell LE, Holmberg M. Tissue-specific splicing of ISCU results in a skeletal muscle phenotype in myopathy with lactic acidosis, while complete loss of ISCU results in early embryonic death in mice. Hum Genet 2011; 129: 371–78. [DOI] [PubMed] [Google Scholar]
- Olsson A, Lind L, Thornell LE, Holmberg M. Myopathy with lactic acidosis is linked to chromosome 12q23.3‐24.11 and caused by an intron mutation in the ISCU gene resulting in a splicing defect. Hum Mol Genet 2008; 17: 1666–72. [DOI] [PubMed] [Google Scholar]
- Paul A, Drecourt A, Petit F, Deguine DD, Vasnier C, Oufadem M et al. . FDXR mutations cause sensorial neuropathies and expand the spectrum of mitocondrial Fe-S-Synthesis Diseases. Am J Hum Genet 2017; 101: 630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul VD, Lill R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim Biophys Acta 2015; 1853: 1528–39. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. Iron-sulfur proteins hiding in plain sight. Nat Chem Biol 2015; 11: 442–5. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak K. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–08. [DOI] [PubMed] [Google Scholar]
- Sheftel AD, Stehling O, Pierik AJ, Netz DJ, Kerscher S, Elsässer HP et al. . Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol Cell Biol 2009; 29: 6059–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Stehling O, Pierik AJ, Elsässer HP, Mühlenhoff U, Webert H et al. . Humans possess two mitochondrial ferredoxins, FDX1 and FDX2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA 2010: 107: 11775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R, Saada A, Halvardson J, Soiferman D, Shaag A, Edvardson S et al. . Deleterious mutation in FDX1L gene is associated with a novel mitochondrial muscle myopathy. Eur J Hum Genet 2014; 22: 902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeweg ME, Ghezzi D, Haack T, Abbink TE, Martinelli D, van Berkel CG et al. . Leukoencephalopathy with thalamus and brainstem involvement and high lactate ‘LTBL’caused by EARS2 mutations. Brain 2012; 135(Pt 5): 1397–94. [DOI] [PubMed] [Google Scholar]
- Stehling O, Paul VD, Bergmann J, Basu S, Lill R. Biochemical analyses of human iron–sulfur protein biogenesis and of related diseases. Methods Enzymol 2018; 599: 227–63. [DOI] [PubMed] [Google Scholar]
- Stehling O, Wilbrecht C, Lill R. Mitochondrial iron–sulfur protein biogenesis and human disease. Biochimie 2014; 100: 61–77. [DOI] [PubMed] [Google Scholar]
- Stevens A. Pigments and minerals. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. Edinburgh: Churchill Livingstone; 1990. p. 245–67. [Google Scholar]
- Webert H, Freibert SA, Gallo A, Heidenreich T, Linne U, Amlacher S et al. . Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat Commun 2014; 5: 5013. [DOI] [PubMed] [Google Scholar]