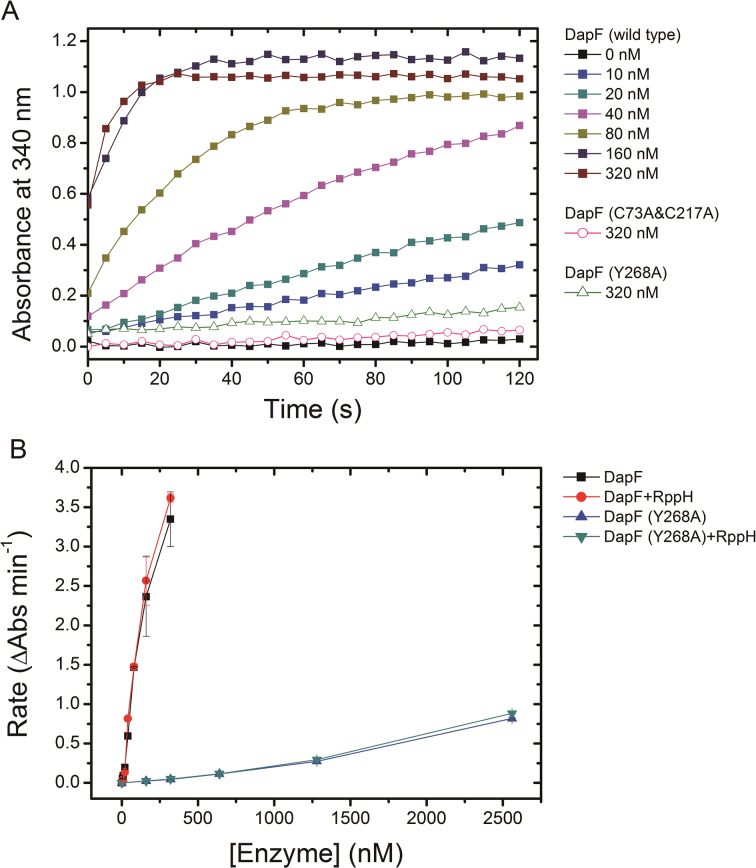
Figure 7.
The effect of RppH on epimerase activity of DapF. (A) The enzymatic activity of DapF was determined using the modified DapF-DAP dehydrogenase coupled spectrophotometric assay. The absorbance at 340 nm (A340) was consecutively recorded for 2 min, with a 5s interval. The filled squares represent A340 of sample including a gradient concentration of wild DapF (ranging from 0 to 320 nM). The pink empty circles represent the active site mutant DapF (C73A&C217A) (320 nM). The green empty triangles are the monomeric mutant DapF (Y268A) (320 nM). (B) The initial change rate of A340 of samples is plotted as a function of DapF concentration, in the presence or absence of RppH. Kinetics were performed at the same conditions for wild-type DapF and the mutant DapF (Y268A). Error bars represent the S.D. of duplicate measurements.