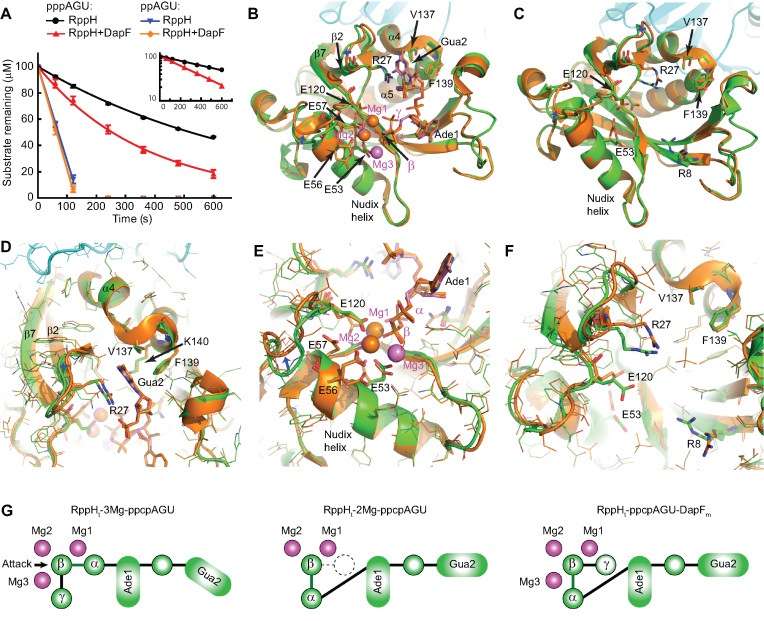
Figure 6.
Identification of potential allosteric interactions of DapF with RppH. (A) Effect of DapF on RppH reactivity with ppAGU and pppAGU RNA substrates, as analyzed by chromatography. pppAGU substrate: RppH alone, black lines and circles; RppH with DapF, red lines and triangles. ppAGU substrate: RppH alone, blue lines and inverted triangles; RppH with DapF, orange lines and diamonds. Error bars represent standard deviations. Inset: the straight lines obtained in semilogarithmic plots show that the reaction of the triphosphorylated substrate proceeded with first-order kinetics. The rate constants with pppAGU were 0.080 ± 0.001 min−1 for RppH and 0.165 ± 0.014 min−1 for the RppH and DapF mixture (average ± SD, n = 2). (B) All-atom superposition of the RppHt–ppcpAGU–DapFm (protein in green, RNA and Mg2+ cations in violet) and RppHt–2Mg–ppcpAGU (orange) structures. Several RNA- and Mg2+-binding residues are shown in sticks. Blue arrows show conformational shifts in the upper part of the catalytic and Gua-2 binding sites. (C) All-atom superposition of the RppH–DapF (green) and RppH (orange) structures. RNA- and Mg2+-binding residues that adopt different conformations in the two structures are shown in sticks. (D and E) Zoomed-in views of the structure superposition (panel B), centered on the Gua2 binding cleft (D) and the active site (E). All residues are shown in lines except residues participating in RNA and Mg2+ recognition, which are in sticks. (F) Zoomed-in view of the structure superposition (panel C), centered on the Gua2 binding cleft and the upper part of the active site. (G) Schematics of RNA 5′-end recognition in different RppH complexes. The left and central panels are for structures from (13), and the right panel is from the current study. Magenta circles, magnesium ions; green circles, phosphates; dashed white circle, structurally disordered γ phosphate; arrow, location of a water molecule poised for nucleophilic attack.