Abstract
In this study, the mechanisms of HuAChE and HuBChE inhibition by Me-P(O)(OPNP)(OR) [PNP = p-nitrophenyl; R = CH2CH3, CH2CH2F, OCH(CH3)2, OCH(CH3)(CH2F)] representing surrogates and fluoro-surrogates of VX and sarin were studied by in vitro kinetics and mass spectrometry. The in vitro measures showed that the VX- and fluoro-VX surrogates were relatively strong inhibitors of HuAChE and HuBChE (ki ~ 105−106 M−1min−1) and underwent spontaneous and 2-PAM-mediated reactivation within 30 min. The sarin surrogates were weaker inhibitors of HuAChE and HuBChE (ki ~ 104−105 M−1min−1), and in general did not undergo spontaneous reactivation, although HuAChE adducts were partially reactivatable at 18 h using 2-PAM. The mechanism of HuAChE and HuBChE inhibition by the surrogates was determined by Q-TOF and MALDI-TOF mass spectral analyses. The surrogate-adducted proteins were trypsin digested and the active site-containing peptide bearing the OP-modified serine identified by Q-TOF as triply- and quadruply-charged ions representing the respective increase in mass of the attached OP moiety. Correspondingly, monoisotopic ions of the tryptic peptides representing the mass increase of the OP-adducted peptide was identified by MALDI-TOF. The mass spectrometry analyses validated the identity of the OP moiety attached to HuAChE or HuBChE as MeP(O)(OR)-O-serine peptides (loss of the PNP leaving group) via mechanisms consistent with those found with chemical warfare agents. MALDI-TOF MS analyses of the VX-modified peptides versus time showed a steady reduction in adduct versus parent peptide (reactivation), whereas the sarin-surrogate-modified peptides remained largely intact over the course of the experiment (24 h). Overall, the presence of a fluorine atom on the surrogate modestly altered the rate constants of inhibition and reactivation, however, the mechanism of inhibition (ejection of PNP group) did not change.

1. Introduction
The use of organophosphorus (OP) compounds as chemical weapons continues to be a significant worldwide threat [1–4]. The main OP compound classes of concern are the V- and G-type chemical warfare agents (CWA) such as VX, sarin, and weaponized insecticide oxons such as paraoxon (PNP = p-nitrophenol). These OPs initiate their toxicity through the inhibition of cholinesterases (ChE’s); acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) (Scheme 1).
Scheme 1.
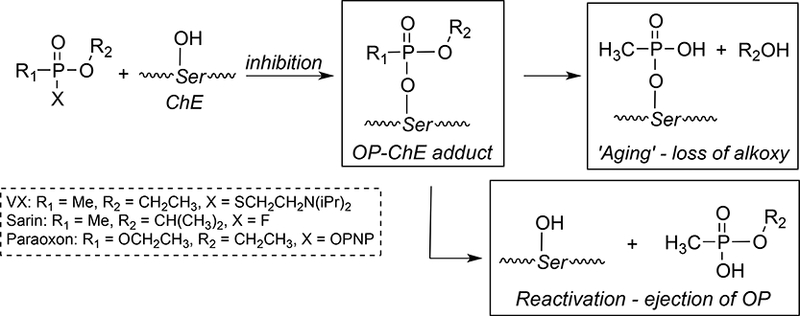
Reaction of organophosphate compounds with acetylcholinesterase.
When AChE is inhibited forming the covalent OP-AChE product, AChE can no longer hydrolyze the neurotransmitter acetylcholine leading to overstimulation of post-synaptic receptors. Even low doses and short exposure times cause a rapid onset of neurotoxicity, and although recovery from serious OP exposures occur, neurotoxicity and neurologic deficits can persist for days to months.[5]
The formation of OP-AChE adducts, which occurs through initial ejection of a leaving group ‘X’ (Scheme 1) is the primary mechanistic trigger for neurotoxicity. Consequently, the formation, stability, and lifetime of the resulting OP-AChE adduct defines its pharmacokinetic (PK) and pharmacodynamic (PD) parameters, which varies greatly with the structure of the phosphoester group R2. Inhibition of BChE occurs via the same mechanism and affords the OP-BChE adduct at serine but is of lesser toxicological consequence. However, the role of BChE as a scavenger of reactive OPs in blood is of critical importance and cannot be overlooked in the overall mechanism of toxic action.
Once the modified OP-ChE is formed, post-inhbitory processes such as aging and reactivation can occur (Scheme 1). Aging occurs via loss of a phosphoester group to afford an irreversibly modified enzyme and is a process favored by an increase in alkyl branching at R2. Reactivation is the process whereby enzyme activity is restored via cleavage of the phospho-serine ester bond and can occur spontaneously (slow) or induced by the addition of oxime antidotes such as 2-pyridine aldoxime (2-PAM) [6–8]. The oxime-mediated process is roughly 100-fold faster than the spontaneous process although the R1 and R2 groups dictate the overall reactivation rate and mechanism.
To more safely study the pharmacological and toxicological implications of exposure to OP chemical warfare agents, the thiolester (VX) or fluoro (sarin) leaving group can be substituted for a p-nitrophenol group (PNP) to decrease the anti-cholinesterase activity, skin penetrability (VX) and volatility (sarin). The use of the p-nitrophenol (PNP) was logically applied from the structure of the potent anti-cholinesterase compound, paraoxon (Table 1). The p-nitrophenoxy analogs of alkylphosphonates (Table 1) were first reported by Fukuto and Metcalf in 1959 [3], and later used as ‘surrogates’ of chemical warfare agents for study [4, 9] including in vivo evaluations [10, 11]. Chambers et al. nicely established that the toxicities of the PNP surrogates followed that of the corresponding nerve agents, cyclosarin > VX > sarin [9] suggesting that the mechanism of cholinesterase inhibition was conserved. In parallel work, Cashman developed surrogates using a thiolester (P(O)-SR) leaving group and adroitly investigated the adduct formation and stereospecificity of action [12, 13] showing they form identical cholinesterase adducts to the CWAs.
Table 1.
Chemical Warfare Agents (CWAs), CWA Surrogates and CWA Fluoro-containing Surrogates and their OP-Cholinesterase Adduct Structures.
| CWA and CWA Surrogates | Structure | Expected OP-Cholinesterase (OP-ChE) Adduct |
|---|---|---|
| VX |  |
|
| VX surrogate | 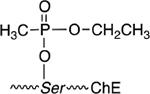 |
|
| F-VX surrogate |  |
|
| SARIN | 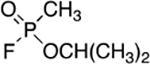 |
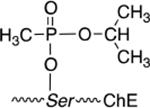 |
| Sarin surrogate |  |
|
| F-Sarin surrogate | 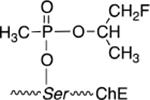 |
|
| PARAOXON (PX) | 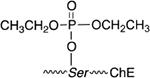 |
The knowledge that surrogates interact with cholinesterases and result in toxicological outcomes similar to CWAs prompted us to prepare the corresponding fluorine atom (18F) positron-emission tomography (PET) tracer analogs in an effort to investigate the pharmacokinetics and pharmacodynamic properties at the molecular level [14–16]. Although the incorporation of a fluorine atom would be expected to alter the chemico-biological properties [14] it is unclear if the presence of the fluorine atom would change the putative mechanistic pathways or the inhibition/post-inhibition rates. Our recent study showed that the F-VX surrogate (Table 1) differs from the non-fluoro analog in inhibition rate and reactivation profile but importantly does not show signs of aging (loss of ethoxy) [14]. This means that the [18F]-fluorine remains part of the OP-serine linkage and attached to acetylcholinesterase (Table 1) for the duration of the tracer half-life (~ 2 h). As a result, the OP tracer could be uniquely beneficial as it can afford quantitative assessment and tissue biodistribution of AChE without aging or aging metabolite confounders. If some or all of the label were lost to aging as [18F]-fluoroethanol, tracer would not be solely associated with AChE and be non-specifically distributed in tissues. In this regard, a detailed evaluation of the mechanism of cholinesterase inhibition by the VX, F-VX, sarin and F-sarin surrogate should also be undertaken and particularly for the sarin analogs since aging is expected to occur to a greater extent. Thus, it is possible to envision [18F]-OP surrogate tracers that could evaluate and quantitate intact OP-AChE (Table 1) or provide a dynamic measure of aging phenomena if it occurs within the tracer half-life.
The present study was designed to assess the mechanism of OP-cholinesterase adduct formation from PNP-based VX and sarin surrogates and their fluoro-(18F) containing analogs F-VX and F-sarin (Table 1) via in vitro kinetics and mass spectrometry. Mass spectrometry has been used extensively to characterize the mechanism of OP-adduct formation with cholinesterases.[13, 17–26] Therefore, to better understand the behavior of CWA surrogates and the corresponding fluoro-surrogates to be used as in vivo PET tracers, the mechanisms of interaction with recombinant human acetyl-(HuAChE) and human butyryl-cholinesterases (HuBChE) were investigated.
1. Materials and Methods.
2.1. General.
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Trypsin was sequencing grade V5111 and purchased from Promega (Madison, WI). C4 ZipTips were obtained from Millipore (Billerica, MA). Recombinant human acetylcholinesterase (HuAChE) from Sigma-Aldrich (C1682; St Lois, MO) and recombinant human serum butyrylcholinesterase (HuBChE) from Lee Biosolutions (Maryland Heights, MO). Serum from Sprague-Dawley rats was purchased from Innovative Research (Novi, MI). Paraoxon was available from prior studies and purified to > 98% on silica gel immediately prior to use. [Note: OPs are hazardous and require gloves and adequate ventilation. OP compounds that come in contact with glassware and plasticware should be immersed in 1.0 M NaOH for decontamination.]
The 1H, 13C and 31P NMR spectra were recorded on a Bruker 400-MHz spectrometer. Chemical shifts are reported in parts per million relative to tetramethylsilane (Me4Si, δ= 0.00 ppm) with CDCl3 as solvent. Small molecule mass spectrometric analyses were conducted using a Micromass LCT - Waters 2795 HPLC - Micromass LCT with 2487 UV Detector (Milford, MA) with caffeine as a molecular weight standard.
2.2. Chemical Syntheses
2.2.1. Syntheses of OP Surrogates of VX, F-VX and sarin.
The organophosphate surrogates of VX, F-VX, sarin and F-sarin [3, 4] were prepared from bis-p- nitrophenyl methylphosphonate and coupling to the requisite alcohol [27] or fluoro-alcohol [16] using DBU. The structures were confirmed using NMR, MS, and comparison to authentic material reported by Fukuto [3] and Meek [4] except fluoro-sarin which is reported herein.
2.2.2. Synthesis of O‐(1‐fluoro-2-propyl)-O-(4-nitrophenyl) methylphosphonate (‘fluoro-sarin surrogate’). (Eqn. 1).
 |
Eqn. 1 |
Solid bis-(O,O-p-nitrophenoxy) methylphosphonate [27] (0.890 g; 2.63 mmol) was added to a solution containing 2.5 mL of anhydrous CH2Cl2. The solution was placed under nitrogen at 25 °C, and to this solution was added 1,8-diazabicycloundec-7-ene (DBU; 430 μL; 0.440 g; 2.9 mmol), whereupon the solution turned yellow-gold (formation of trace p-nitrophenol oxyanion). The solution was stirred at 25 °C for 10–15 min and 1-fluoro-2-propanol (226 mg; 220 μL; 2.9 mmol) was added. Within a minute, the yellow color turned to orange, and TLC showed only the formation of two overlapping spots at Rf ~ 0.20/0.22, EtOAc:hexanes, 3:7) corresponding to product diastereomers and loss of starting material. To check reaction progress, reagents and solvents were gently evaporated, the resultant yellow oil diluted with CDCl3, and the conversion to O-(1-fluoro-2-isopropyl)-O-(p-nitrophenyl) methylphosphonate monitored by 31P NMR. The appearance of diastereomer singlets at 27.7 and 29.2 ppm versus starting material indicated reaction progress. The mixture was diluted with 50 mL CHCl3 and extracted stepwise with 0.1 M HCl (2 × 25 mL), 1 % NaOH (1 × 25 mL), water (25 mL), and sat NaCl (1 × 25 mL). The chloroform layer was dried over sodium sulfate, filtered, and evaporated under a stream of Ar, and placed under high vacuum to afford 662 mg (91%) of pure product free of p-nitrophenol and DBU, with no evidence of starting material. 1H NMR (400.18 MHz, CDCl3) δ 8.25 (dd, J = 17.8 Hz, 2H), 7.42 (dd, J = 9.1 Hz, 2H), 4.95–4.82 (m, 1H), 1.76,1.74 (dd, J = 17.8 Hz), 1.38 (d, J = 6.5 Hz, 1.5H), 1.24 (d, J = 7.1 Hz, 1.5H). 13C NMR (100.6 MHz, CDCh) δ 155.2 (d, Jc-p = 20.1 Hz), 144.6, 125.6, 121.1 (d, Jc-p = 11.0 Hz), 86.0 (d, Jc-p = 24.1 Hz), 84.3 (d, Jc-p = 25.0 Hz), 73.0 and 72.9 (d, Jc-p = 19.0, 26.2 Hz), 17.0 (d, Jc-p = 29.0 Hz), 16.9 (d, Jc-p = 34.0 Hz), 13.0 (d, Jc-p = 35.0 Hz), 11.5 (d, Jc-p = 33.40 Hz). 31P NMR (162 MHz, CDCl3) δ 27.7, 29.2. 19F NMR (376.3 MHz, CDCl3) δ −223.9, −225.1.
2.3. Inhibition and Reactivation of HuAChE and HuBChE.
2.3.1. Determination of inhibition constants (ki).
HuAChE and HuBChE were reconstituted in phosphate-buffered saline (PBS) at pH 7.4 and diluted to make the enzyme activity about 0.5 U/mL. The organophosphate (OP) compounds, VX-, fluoro-VX-, sarin- and fluoro-sarin-surrogates were dissolved in acetonitrile and the aliquots were added to the HuAChE and HuBChE dilutions at 25 °C. After 20 min, enzyme activities were determined using Ellman assay [28]. The inhibition constants (ki) were calculated from the plot of the log-transformed enzyme activity versus the OP concentrations.
2.3.2. Determination of IC50 values.
Rat serum was incubated with 10 μM bambuterol (BChE inhibitor) to assess AChE inhibition [29] or 100 μΜ BW284c51 (AChE inhibitor) to assess BChE inhibition [30] at rt for 15 min and then with OP compounds for 20 min. Enzyme activities were determined using Ellman assay [28]. The IC50 values were calculated by Microsoft Excel Solver with 4-parameter logistic regression in which A is enzyme activity, Amax maximum enzyme activity, Amin minimum enzyme activity.
2.3.3. Determination of Spontaneous and Oxime-Mediated Reactivation Rates.
The procedure was similar that recently reported.[14] HuAChE or HuBChE was diluted in 1 mM asolectin/PBS (pH 7.4) to 0.5 U/mL. A 20.0 mL of the dilution was incubated with 1.0 μM of the OP at 25 °C for a given time to achieve > 90% reduction in activity. A 2.50-mL aliquot of the OP-treated HuAChE or HuBChE was mixed with 250 μL of Ellman reagent with or without the oxime reactivator 2-pyridine-aldoxime methiodide (2-PAM; 10.0 μM) and the absorbance at 412 nm was monitored for the enzyme activity in a 96-well microplate. The change in absorbance at 412 nm versus time was monitored and the rate constant of reactivation (kR) was calculated by from the following equation:
2.4. Trypsin digestion optimization.
Prior to mass spectral analyses, digestion conditions for the cholinesterases and OP-cholinesterase adducts were examined to aid identification of the resultant peptide fragments. Optimal conditions were 20% (v/v) CH3CN, 50 mM sodium phosphate (pH 8.0), 0.05% sodium deoxycholate, 0.5 mg/mL trypsin, at 37 °C for 2 h as aforementioned (section 2.4). Longer digestion times and/or alternate buffers (e.g., 50 mM NH4HCO3 or Na2HPO4; pH 8.0) led to less peptide identifications.
2.5. Analyses by MALDI-TOF and liquid chromatography quadrupole time of flight (LC-Q-TOF) mass spectrometry.
HuAChE or HuBChE were reconstituted in PBS (pH 7.4), incubated with a 100-fold molar excess of the OP compound at rt for 2 h, and trypsin digested at 37°C for 2 h in the buffer containing 20% (v/v) CH3CN, 50-mM sodium phosphate (pH 8.0), 0.05% (w/v) sodium deoxycholate and 0.5 mg/mL trypsin. The digests were then desalted by C4 ZipTip. In general, for MALDI-TOF MS analyses, the sample was spotted on a MALDI target with a-cyano-4-hydroxycinnamic acid as the matrix and analyzed by the MALDI-TOF mass spectrometer (microFlex, Bruker). For LC-Q-TOF MS, the samples were air-dried and reconstituted in 50% acetonitrile in 0.1% formic acid prior to analyses.
For the LC-MS (Q-TOF) analyses, samples of 15 μL each were processed via Dionex Ultimate 3000 nano-UHPLC, with an Acclaim PepMap100 C18 column used for both trapping (300 μm x 5 mm) and final peptide separation. Chromatography solvents were H2O with 0.1% (v/v) formic acid and CH3CN for channel A, and channel B, respectively. Following sample trapping for 2 min at a flow rate of 50 μL/min, the UHPLC valve was switched to elution. From 0 to 2.5 minutes, the elution was 5% B; from 2.5 to 20.0 min elution was linearly changed from 5 to 30% B; from 20 to 23 min, the gradient was ramped from 30 to 95% B; from 23 to 28 minutes, the elution was 95% B; from 28 to 30 min the solvent was linearly ramped from 95% B to 7% B. During the course of the chromatographic run, the loading pump solvent was held at 50 μL/min of 97% water, 3% acetonitrile, and 0.1% formic acid. The liquid chromatography system was connected to a Bruker maXis Impact (Q-TOF) with CaptiveSpray ESI source (resolution ~ 40,000 and accuracy is 1 ppm). Spectra were collected in positive mode from 200 to 2200 m/z at a maximum rate of 2 Hz for both precursor and fragment spectra and with adaptive acquisition time for highly-abundant ions. The resulting data files were processed to select fragmentation spectra with Bruker DataAnalysis and exported as MGF files. SearchGUI (V3.2.17) and Peptideshaker (V1.16.8) were used to identify peptide and peptide adducts.
3. Results and Discussion.
3.1. Reaction of Organophosphonate Surrogates andParaoxon with Cholinesterases.
The OP surrogates and paraoxon (control) were evaluated as inhibitors of HuAChE, HuBChE, and rat serum AChE and BChE (Table 2). The surrogates were all tested as a mixture of stereoisomers to better parallel the use of actual chemical warfare agent, which also exist as mixtures. The serum IC50 values were also conducted to evaluate the OP cholinesterase interaction in the presence of diverse biomolecules for comparison to those assays using pure protein, and to provide estimates of rat dosing levels for OP exposure studies and the corresponding PET tracer experiments. Serum was selected rather than whole blood for analysis to diminish color interferences in the Ellman assay.
Table 2.
Inhibition values for OP surrogates and paraoxona
| OP Compound | HuAChE (ki ×
104) (M−1·min−1) |
HuBChE (ki ×
104) (M−1·min−1) |
Rat Serum AChE IC50 (μM) |
Rat Serum BChE IC50 (μM) |
|---|---|---|---|---|
| VX surrogate | 154±4 | 15.3±1.8 | 1.74±0.12 | 1.98±0.47 |
| F-VX surrogate | 297±56 | 13.7±1.5 | 3.66±0.23 | 4.03±0.73 |
| Sarin surrogate | 7.21±0.36 | 1.31±.09 | 1.63±0.27 | 2.76±0.09 |
|
F-Sarin surrogate |
5.97±0.70 | 1.34±0.09 | 3.79±0.42 | 3.04±0.85 |
| Paraoxon | 628±119 | 144±28 | 0.253±0.11 | 0.189±0.042 |
mean ± SD (n = 3 or 4)
Paraoxon (control) was the most potent inhibitor of HuAChE of those tested displaying ki values at least twice that as F-VX, which was the most potent OP of the surrogates. However, VX- and F-VX-surrogate analogs were potent inhibitors of HuAChE but 2–4 fold less potent than paraoxon. Sarin and fluoro-sarin surrogates were 30- to 50-fold weaker inhibitors of HuAChE than the VX-surrogates and more than 100-fold less potent than paraoxon. Overall, introduction of the fluorine atom led only a minor increase in surrogate inhibitory activity (1.1 to 2-fold), and in some instances had no effect when the distance to the phosphorus reaction center was increased as in F-sarin.
HuBChE was less sensitive to inhibition by the OPs than HuAChE (Table 2). Paraoxon was the best inhibitor of HuBChE and 10- to 100-fold more potent than the VX- and sarin surrogates, respectively. The VX surrogates were 10 to 20-fold less weak inhibitors of HuBChE than HuAChE, whereas the sarin surrogates were 4.5 to 5.5-fold weaker inhibitors. As with HuAChE, the fluorine atom appeared to have no significant effect on the inhibition kinetics of HuBChE. The similar ki values between HuAChE and HuBChE for the sarin surrogates may be due to the greater steric accommodation in the BChE active site.
IC50 values were obtained for rat serum using a selective counter-inhibitor. AChE was assayed after bambuterol BChE inhibition [29] and BChE was assayed after BW284c51 AChE inhibition[30]. The results differed from the analyses using pure enzymes probably because other proteins/enzymes in serum affected the inhibition, non-specific binding and stability of OP compounds. Paraoxon was a potent inhibitor of both AChE and BChE with an IC50 ~ 0.2 μM whereas the surrogates ranged between 1.5 and 4.0 μM. There appeared to be only modest differences in the IC50 values obtained for AChE and BChE by the surrogates with all values within the same order of magnitude and ten-fold less than paraoxon. For the surrogates, the addition of the fluorine atom caused a slight 2-fold decrease in inhibitory potency although BChE did not show any significant difference between sarin and fluoro-sarin surrogate. To assess whether the recombinant protein data aligned with the rat serum data, a Pearson correlation coefficient (r) of the pooled IC50 and Ki values (Table 2) afforded a value of −0.498 indicating a medium to large strength of association [31]. Overall, these results suggest that the presence of a fluorine atom as in the case of a PET tracer would not dramatically affect the reaction with cholinesterases in serum.
3.2. Reactivation of HuAChE and HuBChE Following Inhibition by the VX and F-VX Surrogates.
Each of the OP-enzyme adducts formed from the surrogates and HuAChE or HuBChE were analyzed for their susceptibility to undergo spontaneous or oxime-mediated (2-PAM) reactivation at 30 min and 18 h, which were time points selected to assess rates prior to and following any aging. The fluoro-VX surrogate has been demonstrated to not undergo aging with electric eel AChE (EEAChE) [14] whereas the inhibition of cholinesterases by sarin are known to undergo aging and faster than those formed from VX [32].
The cholinesterase adducts formed from the fluoro-VX surrogate underwent spontaneous reactivation at rates slightly faster than VX-adducted cholinesterases although all were very slow to regain any activity (Table 3). The rate constants of spontaneous reactivation for the cholinesterases formed from the fluoro-VX-surrogate were comparable at the 30 min and 18 h time points but the VX-surrogate adduct formed with BChE was clearly slower to reactivate at 18 h. No measureable enzyme activity was observed for any of the cholinesterases inhibited by the sarin- or fluoro-sarin surrogates presumably due to formation of a more stable OP-ChE structure and/or possible aging.
Table 3.
Spontaneous reactivation rate constants kr (min−1) x10−5
| time | OP compound | HuAChE | HuBChE |
|---|---|---|---|
| 30 min | VX surrogate | 1.38 ± 0.51 | 3.54 ± 0.90 |
| Fluoro-VX surrogate | 5.12 ± 0.75 | 4.36 ± 0.89 | |
| Sarin surrogate | ND | ND | |
| Fluoro-sarin surrogate | ND | ND | |
| 18 h | VX surrogate | 1.31 ± 1.08 | 1.31 ±0.24 |
| Fluoro-VX surrogate | 5.20 ± 1.87 | 7.79 ± 1.49 | |
| Sarin surrogate | ND | ND | |
| Fluoro-sarin surrogate | ND | ND | |
mean ±SD, n = 3; ND = not detectable
Treatment of the HuAChE and HuBChE OP-adducts formed from VX- and fluoro-VX surrogates with 2-PAM led to a 1000-fold increase in reactivation rate as compared to spontaneous reactivation (Table 4). There was no difference between the oxime-mediated rate constants for HuAChE inhibited by VX- or fluoro-VX surrogates at 30 min but at 18 h, the fluoro-VX adduct koxime was two-fold higher. The HuBChE-adducts formed from the fluoro-VX surrogate had rate constants about two-fold faster than those inhibited by the VX-surrogate at 30 min and 18 h time points. Although statistically relevant reactivation rates could not be determined above background at 30 min following inhibition of HuAChE or HuBChE by the sarin- or fluoro-sarin surrogates by 18 h the rate could be observed and measured accurately. The rate constants of oxime reactivation were similar for the sarin- and fluoro-sarin inhibited enzyme, and slightly higher than those obtained from the cholinesterases inhibited by the VX-surrogates suggesting that these OP-enzyme complexes did not undergo aging to an appreciable extent. Therefore, the analogous fluorine-18 OP PET tracer could be expected to modify these enzymes via ejection of PNP (Scheme 1), and remain intact until reactivation would release MeP(O)(OCH2CH2F)(OH).
Table 4.
Oxime Reactivation Rate Constants koxime (min−1) ×10−2
| Time | OP Compound | HuAChE | HuBChE |
|---|---|---|---|
| 30 min | VX surrogate | 4.43 ± 0.39 | 1.54 ± 0.24 |
| Fluoro-VX surrogate | 4.36 ± 1.73 | 3.29 ± 0.52 | |
| Sarin surrogate | ND | ND | |
| Fluoro-sarin surrogate | ND | ND | |
| 18 h | VX surrogate | 1.20 ± 0.11 | 1.33 ± 0.38 |
| Fluoro-VX surrogate | 2.63 ± 0.98 | 2.05 ± 1.12 | |
| Sarin surrogate | 6.64 ± 1.22 | 6.03 ± 0.23 | |
| Fluoro-sarin surrogate | 5.43 ± 0.64 | 7.86 ± 0.20 | |
mean ± SD, n=3; NA = not determined (no appreciable rate measured)
In general, the presence of a fluorine atom on the surrogates caused a slight increase in the spontaneous and oxime-mediated reactivation rate constants that may be due to a favorable electron-withdrawing moiety role toward the nucleophilic action of water or oximate anion of 2-PAM.
3.3. Identification of Surrogate-Cholinesterase Adducts by Mass Spectrometry.
To further delineate the mechanism of inhibition, quadrupole-time of flight (Q-TOF) and MALDI-TOF MS analyses were conducted with tryptic active-site peptides obtained following reaction of HuAChE or HuBChE modified by VX-, fluoro-VX-, sarin- and fluoro-sarin surrogates. The use of MALDI-TOF MS analyses in particular has proven valuable in the elucidation of OP-cholinesterase adducts and mechanistic pathways [13, 17–20], as the conditions for Q-TOF ionization can sometimes limit characterization [25].
3.3.1. Identification of OP-ChE Adducts by Quadrupole-Time of Fight (Q-TOF) Mass Spectrometry.
HuAChE or HuBChE were inhibited with a 100× molar ratio of the OP surrogate at rt for 2 h, trypsin digested, and desalted by C4 ZipTip. The resultant peptide fragments were identified from the extracted ion chromatogram using SearchGUI (V3.2.17) and Peptideshaker (V1.16.8). Unmodified enzyme was digested and the peptide containing the active site serine (Table 5; S) identified as a control. Due to differences in cleavage sites, the tryptic peptide derived from HuAChE containing the active site serine shows a much higher mass ion (m/z 4268) [20] than that of HuBChE (m/z 2929).
Table 5.
Mass ions identified for unmodified and OP-modified tryptic peptides from HuAChE and HuBChE.
|
Peptide (A or B)
[Ser]-OP(O)XY ‘OP-adducted peptide’ |
Mass of
peptide or OP-peptide adduct |
OP-Adduct Identified
as Multiply-charged Ion |
|---|---|---|
| HuAChE-peptide-OH | 4268.1445 | 1423.7148 (M3+) |
| A-OP(O)(CH3)(OCH2CH3) | 4374.1628 | 1459.0543 (M3+) |
| A-OP(O)(CH3)(OCH2CH2F) | 4392.1534 | 1465.0511 (M3+) |
| A-OP(O)(CH3)(OCH(CH3)2) | 4388.1785 | 1098.5647 (M4+) |
| A-OP(O)(CH3)(OCH(CH3)(CH2F)) | 4406.1691 | 1469.7178 (M3+) |
| HuBChE-peptide-OH | 2928.5214 | 977.1738 (M3+) |
| B-OP(O)(CH3)(OCH2CH3) | 3034.5398 | 1012.5132 (M3+) |
| B-OP(O)(CH3)(OCH2CH2F) | 3052.5303 | 1018.5101 (M3+) |
| B-OP(O)(CH3)(OCH(CH3)2) | 3048.5554 | 1017.1910 (M3+) |
| B-OP(O)(CH3)(OCH(CH3)(CH2F)) | 3066.5460 | 1022.8334 (M3+) |
A = LALQWVQENVAAFGGDPTSVTLFGESAGAASVGMHLLSPPSR
B = SVTLFGESAGAASVSLHLLSPGSHSLFTR
Inhibition of HuAChE and HuBChE by the OP-surrogates affords the covalently modified OP-peptide adduct structures (Table 1) that correlate with an increase in mass (Table 5). The monoisotopic ions would display the VX-surrogate modified peptide with a net mass increase by m/z 106, fluoro-VX surrogate by m/z 124, sarin-surrogate by m/z 120, and fluoro-sarin surrogate by m/z 138. Mass spectral analysis of each of the trypsin digests led to positive identification of the OP-adduct, however, the OP-modified peptides were all identified as triply-charged ions except the sarin-surrogate modification of HuBChE, which was identified as a quadruply-charged ion (Table 5).
The identification of identical OP-adducts for both HuAChE and HuBChE from the VX-analogs further supports a conserved mechanism of inhibition by the surrogates with displacement of the p-nitrophenol leaving group, and identification of intact (not aged) OP-cholinesterase.
Detailed interrogation of the mass spectra revealed no evidence of aging (loss of alkoxy group). Although AChE/BChE inhibited by the VX-surrogates were not expected to result in aged products, inhibition by sarin and sarin analogs undergo aging although the process is time dependent. One reason that OP-cholinesterase aging peptides are not observed by mass spectrometry is facile betaelimination of the formed phosphoanion [26] from the digest and/or the LCMS ionization conditions. In sum, the Q-TOF analyses showed formation of the neutral, OP-cholinesterase peptides modified covalently at the active site serine for each surrogate but the products of aging could not be readily identified in these experiments.
3.3.1. Identification of OP-ChE Adducts by MALDI-TOF Mass Spectrometry.
The inhibition, digestion and desalting were accomplished via a method identical to that for the Q-TOF analyses. The sample was spotted on a MALDI target with α-cyano-4-hydroxy cinnamic acid as the matrix and analyzed by the MALDI-TOF mass spectrometer (microFlex, Bruker) (Fig. 1; Table 6). Each of the OP-modified peptides from inhibition of HuAChE were clearly identified as their monoisotopic ions (Fig. 1), and in agreement with the Q-TOF study, there was no evidence of any aging. We were unable to observe any ions associated with the inhibition of HuBChE by the OP surrogates. Additional peaks observed in the spectra appeared at 3802/3803 and 3582 that are attributed to the tryptic peptide FSFVPVVDGDFLSDTPEALINAGDFHGLQVLVGVVK and an unidentified yet consistent peak in the recombinant HuAChE digest that was used as internal standard. To further investigate if the trypsin cleavage sites or matrix conditions led to peptides that were not amenable to MALDI-TOF MS analysis, digestion with chymotrypsin was also evaluated. However, no mass ions correlating with the OP inhibition were found for either HuAChE or HuBChE. Although the digests were complete, the MS spectra were too complex for identification of single ions or overall deconvolution.
Figure 1.

Identification of Tryptic Active Site Peptides of OP-modified Human Acetylcholinesterase (HuAChE) Using MALDI-TOF Mass Spectrometry
Table 6.
Monoisotopic mass ions identified for unmodified and OP-modified tryptic peptides from HuAChE.
|
HuAChE
[Ser]-OP(O)XY ‘OP-adducted peptide’ |
Expected m/z | Observed m/z |
Deviation (ppm) |
|---|---|---|---|
| HuAChE-peptide-Ser-OH | 4268.1445 | 4268.1868 | 9.9 |
| A-OP(O)(CH3)(OCH2CH3) | 4423.1718 | 4423.2200 | 10.9 |
| A-OP(O)(CH3)(OCH2CH2F) | 4374.1628 | 4374.2185 | 12.7 |
| A-OP(O)(CH3)(OCH(CH3)2) | 4392.1534 | 4392.1771 | 5.4 |
| A-OP(O)(CH3)(OCH(CH3)(CH2F)) | 4388.1785 | 4388.1829 | 1.0 |
A = LALQWVQENVAAFGGDPTSVTLFGESAGAASVGMHLLSPP
3.3. Monitoring OP-modified HuAChE Reactivation Using MALDI MS.
Initially we observed that the ratio of the peaks assigned to F-VX-modified peptide and native peptide was greatest at 4 h, and that by 24 h the ratio had diminished to near zero. This observation suggested that the amount of OP-adducted peptide that had reactivated could be measured versus time and report the amount of spontaneous reactivation. In this experiment, the activity of HuAChE was inhibited to greater than 90% (less than 10% activity remaining) by the surrogates, and the ratio of peak areas of adducted peptides to intact (unmodified) peptide measured and plotted at several time-points up to 24 h for each of the surrogates.
For inhibition of HuAChE by the VX and F-VX surrogates, the ratio of adducted-peptide to unmodified peptide grew to a maximum of 6–7% after a few hours and then decreased over time up to 24 h (Fig. 2). The ratio of F-VX adducted peptide decreased to a much greater extent than the VX-modified peptide within 24 h consistent with our prior report that reactivation occurs more readily with the fluoro analog.[14] In both instances, the OP-enzyme peptides formed from VX-surrogates decreased steadily over time after an initial formation of maximum OP-adduct.
Figure 2.

Plots of peak area ratio (adducted/unmodified) versus time showing the amount of reactivation of VX-(panel A) and F-VX-modified (panel B) HuAChE as a ratio to unmodified (intact) peptide.
When the same experiment was carried out with the sarin-and fluoro-sarin surrogates, the ratio of OP-peptide to unmodified peptide did not decrease appreciably after the initial inhibition ratio achieved a maximum of 5–6% (within an hour) (Fig 3).
Fig 3.
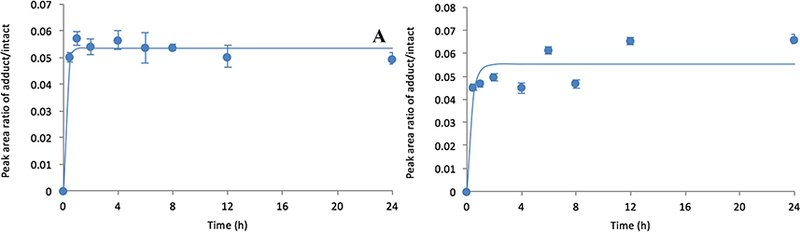
Plots of peak area ratio (adducted/unmodified) versus time leading to reactivation of sarin-(panel A) and F-sarin-modified (panel B) HuAChE.
Therefore, there was little to no spontaneous reactivation observed up to 24 h for the sarinsurrogate experiments, which was expected since reactivation of an OP-modified enzyme bearing the more sterically congested isopropoxy groups would reactivate to a lesser extent than that of the corresponding VX surrogates containing an ethoxy moiety. Moreover, the possibility of aging cannot be excluded for the sarin-modified HuAChE even though the aged peptide adduct could not be observed by MS. Of course, it is unclear whether this non-reactivation mechanism would be observed in vivo.
4. Conclusions.
This study showed that the VX- and sarin-surrogates modify AChE and BuChE via mechanisms that afford OP-enzyme structures that are identical to the CWA. Specifically, all of the surrogates modified acetyl- and butyrylcholinesterase by ejection of the p-nitrophenol (PMP) leaving group. The surrogates differ in inhibition rate constants but in an expected order of reactivity. It was also demonstrated that attachment of a fluorine atom to the surrogates does not dramatically affect the inhibition-reactivation mechanisms for HuAChE and HuBChE yet plays a significant role in the reaction kinetics. Mass spectrometry experiments using Q-TOF and MALDI-TOF validated the mechanism of inhibition for each of the surrogates as loss of the PNP group, although there were limitations in observing certain adducts formed between the surrogates and HuBChE. In novel experiments, it was shown that the time course of HuAChE reactivation could be assessed by MALDI MS following inhibition using a ratio of unmodified to modified peak areas. Herein, HuAChE inhibited by the VX-surrogates showed a steady and measureable reactivation whereas inhibition by the sarin surrogates showed nearly no reactivation up to 24 h. Collectively, the experiment presented point to a conserved mechanism of inhibition by the OP and fluorine-containing OP surrogates with loss of the PNP group but importantly, the presence of the fluorine atom does not alter the mechanism. In fact, when present the fluorine atom appears to stabilize the covalently modified OP-cholinesterase adduct leading to a preference for reactivation versus aging. This would be expected in cases where aging occurs via a cationic mechanism as the electron-withdrawing fluorine would suppress nearby formation of electron deficient centers.[33]
Supplementary Material
Highlights.
Fluoro-containing, p-nitrophenyl surrogates of VX and sarin inhibited HuAChE and HuBChE.
VX-surrogates were more potent inhibitors than sarin-surrogates.
Cholinesterase adducts formed from the VX-surrogates were reactivated.
The mechanism of inhibition was elucidated by Q-TOF and MALDI-TOF mass spectrometry.
The time course of VX-modified cholinesterase reactivation was monitored using MALDI-TOF MS.
5. Acknowledgements.
Supported by the CounterACT Program, Office of the Director, National Institutes of Health (NIH) and the National Institute of Neurological Disorders and Stroke (NINDS) Award Number U01 NS092495. Funding for the Proteomics, Metabolomics and Mass Spectrometry Facility was made possible in part by the MJ Murdock Charitable Trust and the NIGMS of the NIH award number P20GM103474. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- OP
organophosphate
- ChE
cholinesterase
- HuAChE
recombinant human acetylcholinesterase
- HuBChE
recombinant human butyrylcholinesterase
- MS
mass spectrometry
- MALDI
matrix-assisted laser desorption/ionization
- Q-TOF
quadrupole time of flight
- VX
ethyl ((2-[bis(propan-2-yl)amino]ethyl)sulfanyl)(methyl)phosphonate
- sarin
(RS)-O-Isopropyl methylphosphonofluoridate
- F-VX
fluoro-VX surrogate
- F-sarin
fluoro-sarin surrogate
Footnotes
6. Conflict of interest
No conflicts of interest are reported by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barthold CL, Schier JG, Organic phosphorus compounds--nerve agents, Crit Care Clin, 21 (2005) 673–689, v-vi. [DOI] [PubMed] [Google Scholar]
- [2].Morita H, Neurotoxicity of nerve agents, Brain and Nerve, 47 (1995) 1129–1134. [PubMed] [Google Scholar]
- [3].Fukuto TR, Metcalf RL, The Effect of Structure on the Reactivity of Alkylphosphonate Esters, Journal of the American Chemical Society, 81 (1959) 372–377. [Google Scholar]
- [4].Meek EC, Chambers HW, Coban A, Funck KE, Pringle RB, Ross MK, Chambers JE, Synthesis and In Vitro and In Vivo Inhibition Potencies of Highly Relevant Nerve Agent Surrogates, Toxicological Sciences, 126 (2012) 525–533. [DOI] [PubMed] [Google Scholar]
- [5].Deshpande LS, Carter DS, Phillips KF, Blair RE, DeLorenzo RJ, Development of status epilepticus, sustained calcium elevations and neuronal injury in a rat survival model of lethal paraoxon intoxication, Neurotoxicology, 44 (2014) 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fest C, Schmidt KJ, The chemistry of organophosphorus pesticides; reactivity, synthesis, mode of action, toxicology, Springer; Verlag, Berlin, New York, 1973. [Google Scholar]
- [7].Fukuto TR, Mechanism of action of organophosphorus and carbamate insecticides, Environ. Health Perspect, 87 (1990) 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gallo MA, Lawryk NJ, Organic Phosphorus Pesticides, Academic Press, San Diego, 1991. [Google Scholar]
- [9].Coban A, Carr RL, Chambers HW, Willeford KO, Chambers JE, Comparison of inhibition kinetics of several organophosphates, including some nerve agent surrogates, using human erythrocyte and rat and mouse brain acetylcholinesterase, Toxicol Lett, 248 (2016) 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chambers JE, Chambers HW, Meek EC, Pringle RB, Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates, Chemico-biological interactions, 203 (2013) 135–138. [DOI] [PubMed] [Google Scholar]
- [11].Chambers JE, Meek EC, Chambers HW, Novel brain-penetrating oximes for reactivation of cholinesterase inhibited by sarin and VX surrogates, Annals of the New York Academy of Sciences, 1374 (2016) 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barakat NH, Zheng X, Gilley CB, MacDonald M, Okolotowicz K, Cashman JR, Vyas S, Beck JM, Hadad CM, Zhang J, Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase, Chem Res Toxicol, 22 (2009) 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilley C, MacDonald M, Nachon F, Schopfer LM, Zhang J, Cashman JR, Lockridge O, Nerve agent analogues that produce authentic soman, sarin, tabun, and cyclohexyl methylphosphonate-modified human butyrylcholinesterase, Chem Res Toxicol, 22 (2009) 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chao CK, Ahmed SK, Gerdes JM, Thompson CM, Novel Organophosphate Ligand O-(2-Fluoroethyl)-O-(p-Nitrophenyl)Methylphosphonate: Synthesis, Hydrolytic Stability and Analysis of the Inhibition and Reactivation of Cholinesterases, Chem Res Toxicol, 29 (2016) 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].James SL, Ahmed SK, Murphy S, Braden MR, Belabassi Y, VanBrocklin HF, Thompson CM, Gerdes JM, A novel fluorine-18 beta-fluoroethoxy organophosphate positron emission tomography imaging tracer targeted to central nervous system acetylcholinesterase, ACS Chem Neurosci, 5 (2014) 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Neumann KD, Thompson CM, Blecha JE, Gerdes JM, VanBrocklin HF, An improved radiosynthesis of O-(2-[18 F]fluoroethyl)-O-(p-nitrophenyl)methylphosphonate: A first-in-class cholinesterase PET tracer, J Labelled Comp Radiopharm, 60 (2017) 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doorn JA, Gage DA, Schall M, Talley TT, Thompson CM, Richardson RJ, Inhibition of acetylcholinesterase by (1S,3S)-isomalathion proceeds with loss of thiomethyl: kinetic and mass spectral evidence for an unexpected primary leaving group, Chem Res Toxicol, 13 (2000) 1313–1320. [DOI] [PubMed] [Google Scholar]
- [18].Elhanany E, Ordentlich A, Dgany O, Kaplan D, Segall Y, Barak R, Velan B, Shafferman A, Resolving pathways of interaction of covalent inhibitors with the active site of acetylcholinesterases: MALDI-TOF/MS analysis of various nerve agent phosphyl adducts, Chem Res Toxicol, 14 (2001) 912–918. [DOI] [PubMed] [Google Scholar]
- [19].Jennings LL, Malecki M, Komives EA, Taylor P, Direct analysis of the kinetic profiles of organophosphate-acetylcholinesterase adducts by MALDI-TOF mass spectrometry, Biochemistry, 42 (2003) 11083–11091. [DOI] [PubMed] [Google Scholar]
- [20].Mangas I, Taylor P, Vilanova E, Estevez J, Franca TC, Komives E, Radic Z, Resolving pathways of interaction of mipafox and a sarin analog with human acetylcholinesterase by kinetics, mass spectrometry and molecular modeling approaches, Arch Toxicol, 90 (2016) 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carletti E, Li H, Li B, Ekstro?m F Nicolet Y, Loiodice M, Gillon E, Froment MT, Lockridge O, Schopfer LM, Masson P, Nachon F, Aging of cholinesterases phosphylated by tabun proceeds through O-dealkylation, Journal of the American Chemical Society, 130 (2008) 16011–16020. [DOI] [PubMed] [Google Scholar]
- [22].Grigoryan H, Schöpfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O, Mass spectrometry identifies multiple organophosphorylated sites on tubulin, Toxicol Appl Pharmacol, 240 (2009) 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O, Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents, Chem Biol Interact, 175 (2008) 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O, Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry, Toxicol Sci, 83 (2005) 303–312. [DOI] [PubMed] [Google Scholar]
- [25].Spaulding RS, George KM, Thompson CM, Analysis and sequencing of the active-site peptide from native and organophosphate-inactivated acetylcholinesterase by electrospray ionization, quadrupole/time-of-flight (QTOF) mass spectrometry, J Chromatogr B Analyt Technol Biomed Life Sci, 830 (2006) 105–113. [DOI] [PubMed] [Google Scholar]
- [26].Thompson CM, Prins JM, George KM, Mass spectrometric analyses of organophosphate insecticide oxon protein adducts, Environ Health Perspect, 118 (2010) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tawfik DS, Eshhar Z, Bentolila A, Green BS, 1,8-Diazabicyclo[5.4.0]undecene Mediated Transesterification of p-Nitrophenyl Phosphonates: A Novel Route to Phosphono Esters, Synthesis, 1993 (1993) 968–972. [Google Scholar]
- [28].Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM, A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem Pharmacol, 7 (1961) 88–95. [DOI] [PubMed] [Google Scholar]
- [29].Bosak A, Gazić I, Vinković V, Kovarik Z, Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers, Chemico-Biological Interactions, 175 (2008) 192–195. [DOI] [PubMed] [Google Scholar]
- [30].Radic Z, Pickering NA, Vellom DC, Camp S, Taylor P, Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors, Biochemistry, 32 (1993) 12074–12084. [DOI] [PubMed] [Google Scholar]
- [31].Fulekar MH, Bioinformatics : applications in life and environmental sciences, Springer; New Delhi : Capital Publishing, Dordrecht, New York, 2009. [Google Scholar]
- [32].Worek F, Aurbek N, Wetherell J, Pearce P, Mann T, Thiermann H, Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: Pig versus minipig acetylcholinesterase, Toxicology, 244 (2008) 35–41. [DOI] [PubMed] [Google Scholar]
- [33].Quinn DM, Topczewski J, Yasapala N, Lodge A, Why is Aged Acetylcholinesterase So Difficult to Reactivate?, Molecules, 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


