Figure 2. Study diagram of the clinical study.
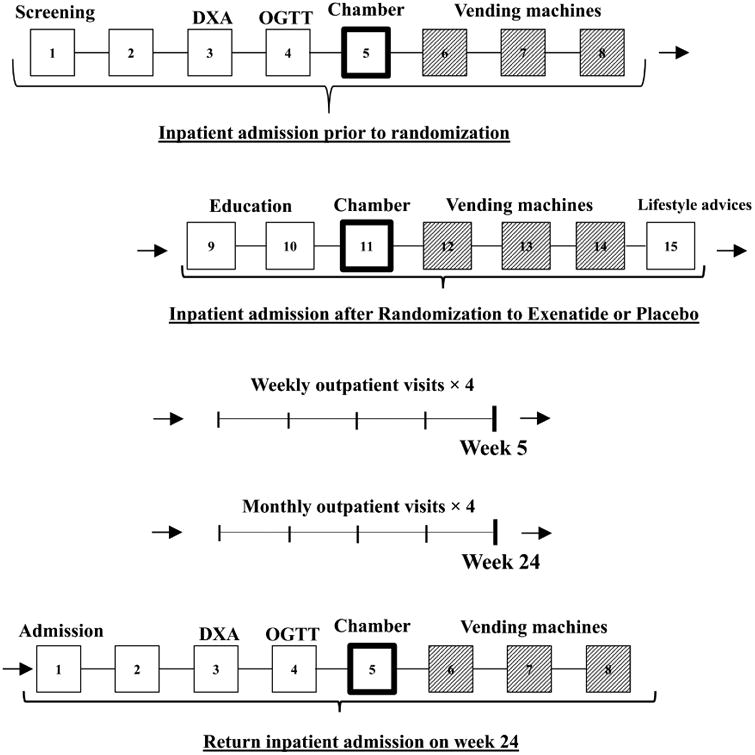
We divided the outline of the study in 2 portions (described in detail below): first inpatient stay (composed by inpatient admission prior to randomization and inpatient admission after randomization) and second (return) inpatient stay at week 24.
Inpatient Admission prior to randomization: Day 1: Screening interview including screening labs and informed consent
Day 3: DXA (dual energy X-ray absorptiometry)
Day 4: OGTT (oral glucose tolerance test)
Day 5: Whole room indirect calorimetry for the assessment of the 24h energy expenditure at baseline
Day 6-7-8: Assessment of ad libitum food intake with vending machine paradigm at baseline
Inpatient Admission after randomization: Day 9-10: Exenatide/Placebo injection education (subcutaneous)
Day 11: Whole room indirect calorimetry for the assessment of the 24h energy expenditure during Exenatide/Placebo
Day 12-13-14: Assessment of ad libitum food intake with vending machine paradigm during intervention period
Day 15: Lifestyle recommendations
After the first 2 weeks of inpatient visit, volunteers returned for weekly follow up visit up to 5 weeks, and then, for monthly follow up visits up to week 24
Return Inpatient Admission on week 24: Day 1: Screening interview including screening labs and informed consent
Day 3: DXA (dual energy X-ray absorptiometry)
Day 4: OGTT (oral glucose tolerance test)
Day 5: Whole room indirect calorimetry for the assessment of the 24h energy expenditure at baseline
Day 6-7-8: Assessment of ad libitum food intake with vending machine paradigm at baseline
