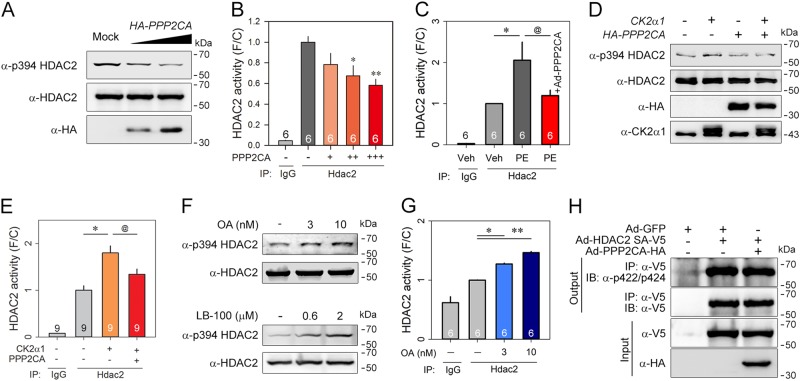
Fig. 2. PPP2CA specifically regulates both HDAC2 phosphorylation and subsequent activation.
a PPP2CA reduced HDAC2 S394 phosphorylation. HDAC2 phosphorylation was decreased in a PPP2CA-HA dose-dependent manner in H9c2 cells, a cardiomyoblast cell line. b The intrinsic activity of HDAC2 was decreased in a PPP2CA dose-dependent fashion. c Adenovirus expressing PPP2CA (Ad-PPP2CA) abridged HDAC2 hyperactivation induced by treatment with 20 μmol/L phenylephrine (PE), an alpha-adrenergic agonist, in NRVCs. PE-induced enzymatic activation of HDAC2 was significantly diminished by simultaneous infection of PPP2CA. The changes were displayed as the fold change by dividing by the mean value of the control group. PPP2CA attenuated HDAC2 phosphorylation and thereby activation induced by CK2α1. d, e CK2α1-induced HDAC2 phosphorylation (d, 2nd lane) and HDAC2 activation (e, 2nd bar) were completely blocked by co-transfection of PPP2CA (e, 4th lane, and e, 4th bar) in H9c2 cells. Okadaic acid (OA), a selective PP2A inhibitor, induced an increase in phosphorylation (f, upper panel) and intrinsic activity (g) of HDAC2. LB-100, an alternate PP2A inhibitor, also induced HDAC2 S394 phosphorylation (f, lower panel). h PPP2CA specifically regulated S394 phosphorylation of HDAC2. PPP2CA failed to reduce phosphorylation of S422 and S424 phosphorylation in the HDAC2 S394A mutant. The designated number in (b) and (c) indicated an independent experimental set. Data are presented as the mean ± SEM. * and @ indicate p < 0.05. In case of **p < 0.01