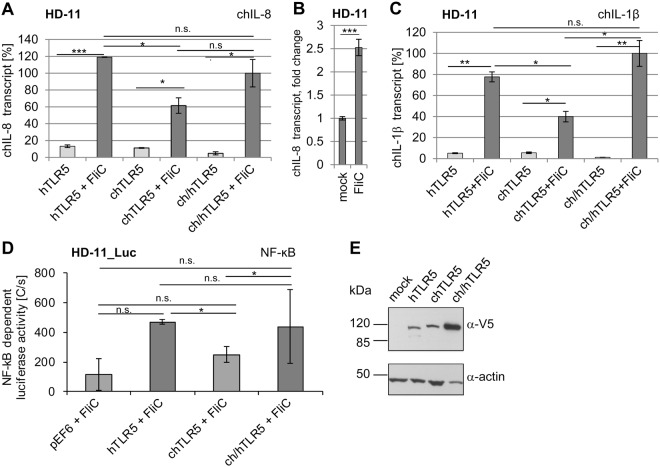
Figure 5.
Reciprocal expression and functionality of human, chicken and chimeric TLR5 within chicken cellular background. Chicken HD-11 (A,B,C and E) or NF-κB reporter HD-11 cells (D) were nucleofected with expression plasmids coding for human (h), chicken (ch) or chimeric (ch/h) TLR5 from different species as indicated in the Methods. 24 h post nucleofection, selected wells were coincubated with purified recombinant Salmonella FliC (200 ng/well in 6-well plate format or 25 ng FliC in 96-well plate format). For quantitative RT-PCR, cells were stimulated for 30 min, and transcript amounts of chicken IL-8 (A,B) and IL-1β (C) were determined. Quantitated transcript values were normalized to respective chicken GAPDH transcript amounts and are presented in [%] relative to FliC-activated ch/hTLR5, set to 100%. In panel B, chIL-8 transcript induction in FliC-activated versus mock-coincubated non-transfected HD-11 cells is shown as fold induction. This result demonstrates a significant but considerably lower response of intrinsic chTLR5 in comparison to the activities provided by transfected TLR5 expression constructs shown in A and C. NF-κB-dependent luciferase activity was measured after 3.5 h of HD-11_luc stimulation using SteadyGlo luciferase assay (D). In D, the respective control values for all constructs (pEF6-V5 empty, hTLR5, chTLR5, ch/hTLR5) without FliC activation were subtracted from each FliC-activated value as background to yield the depicted activated end values for each construct in luminescence photon counts [C per s]. Mean and standard deviation from technical duplicates for transcriptional activation (A,C) and from technical triplicates for intrinsic TLR5 activity in HD-11 (B) and NF-κB-dependent activation (D) are shown. All experiments were independently repeated at least once, with similar outcomes. Significant differences between specific conditions are indicated by lines and asterisks in A, B, C and D (Student’s t-test; *0.01 < p < 0.05; ***p < 0.001; n.s non significant). Western Blot analysis for expression of TLR5 constructs using cleared lysates of nucleofected HD-11 cells (E). Immunoblotting was performed using anti-V5 or anti-actin antibody (loading control).