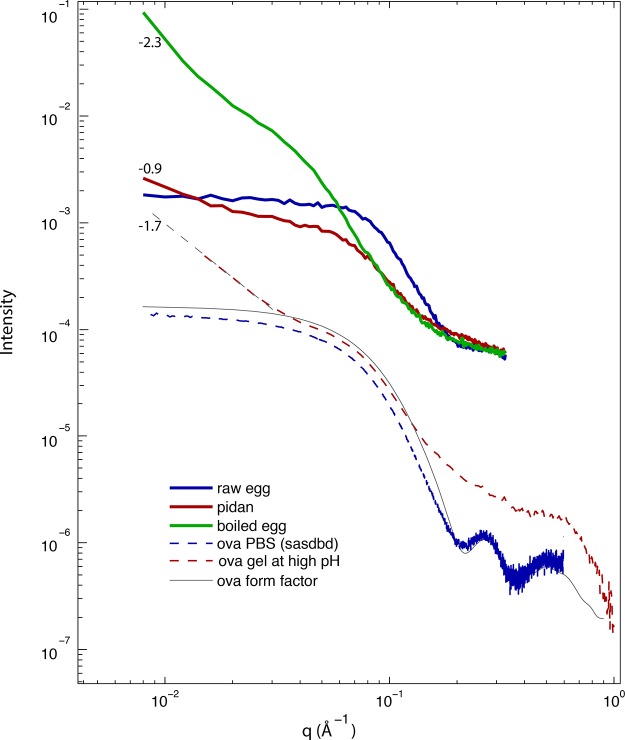
Figure 3.
Small- and wide-angle X-ray scattering (SAXS/WAXS) from egg white and ovalbumin, I(q) for all liquid and gel materials described in this study. Curves are as follows: blue curve, raw egg white; red curve, traditional pidan; green curve, boiled egg; dashed red curve, alkaline ovalbumin gel; dashed blue curve, ovalbumin from SASBDB; gray curve, ovalbumin form factor calculated from crystal structure 1OVA. Numeric labels show the log–log slope of those data for q < 3 × 10–2 Å–1, providing an estimate of the fractal dimension of a material at length scales larger than a protein monomer. In pidan, the absence of the peak at q ∼ 0.25 Å–1 that is present in native ovalbumin suggests that the high-base treatment induces partial loss of protein secondary structure. The low-q log–log slopes of pidan and high-base ovalbumin at q < 0.04 Å–1 (slope from −0.6 to −1.7) are intermediate between those of liquid egg white and an ovalbumin at neutral pH (|slope| ≪ 1) and boiled egg (slope ∼−2.3), indicating that pidan protein forms a network with large-scale structure with a lower fractal dimension than the gelled boiled egg material.