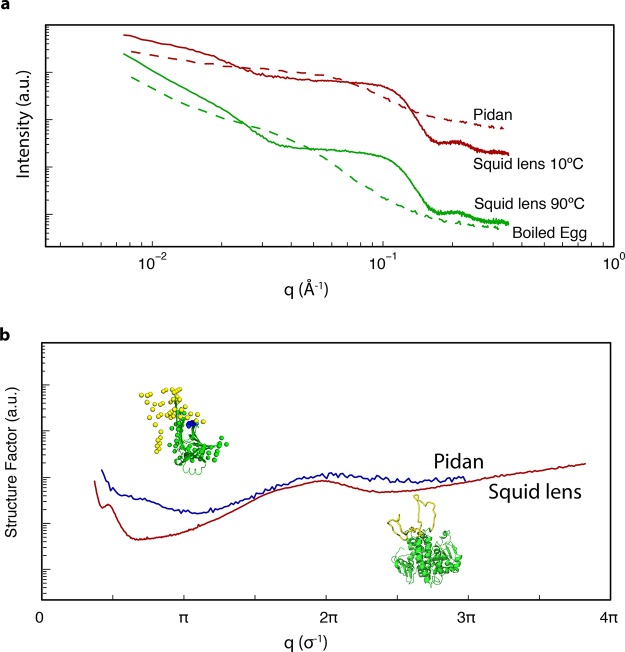
Figure 7.
Hypothetical structural origins of patchy thermodynamics in S-Crystallin and pidan proteins. (a) I(q) from SAXS for pidan (red dashed line) and boiled egg white (green dashed line, this study), and squid lens at 10 °C (red solid line) and 90 °C (green solid line, lens data from ref (2)). In both the squid and the egg system, the slope at low values of q becomes sharper upon heating to near-boiling, while the overall shape of the curve does not change at high values of q. (b) Structure factors of pidan (blue curve) and squid lens (red curve), normalized to the diameter of an individual monomer (σ). Both structure factors show a peak at the diameter of the protein (2π/σ), indicating frequent pairwise interactions; a minimum at π/σ, consistent with reduced density fluctuation at distances of a few σ, and a log−log slope of ∼−2.5 at q ≤ 0.5π/σ. Insets show predicted structures of squid S-Crystallin proteins, and ovalbumin in the high-base state that induces patchy-particle thermodynamics. Our hypothesis about the regions of protein structure responsible in both systems for hard-sphere interaction is shown in green, and the regions inferred to be responsible for patchy attractive interactions are shown in yellow.