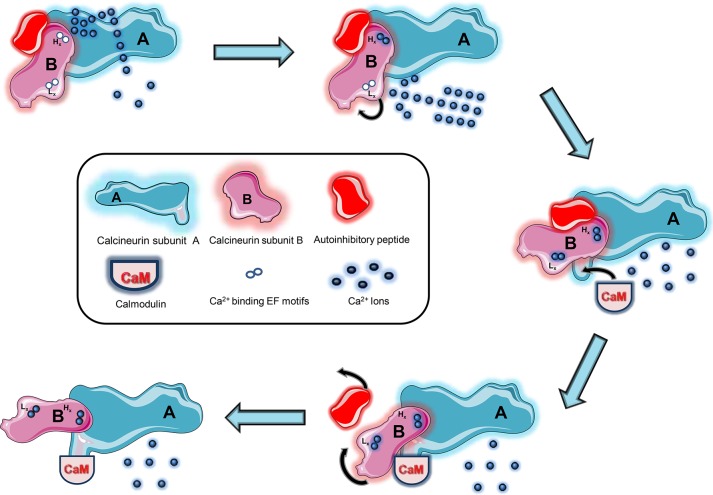
Figure 1.
Activation of calcineurin (CaN). The phosphatase is activated through the sequence of two subsequent conformations. Initially, the influx of calcium (Ca2+) raises the intracellular calcium load. At the nanomolar range, Ca2+ first binds to the two higher-affinity EF motifs (Hx) of subunit B, tightly adhering subunit B to subunit A and stabilizing the enzyme. As Ca2+ levels rise to the micromolecular threshold, it binds to the lower-affinity EF motifs (Lx), spurring a conformational change that unveils the calmodulin (CaM) site to which CaM binds and initiates a second conformational change responsible for displacing the autoinhibitory peptide. The final conformation stabilizes and fully renders CaN active.