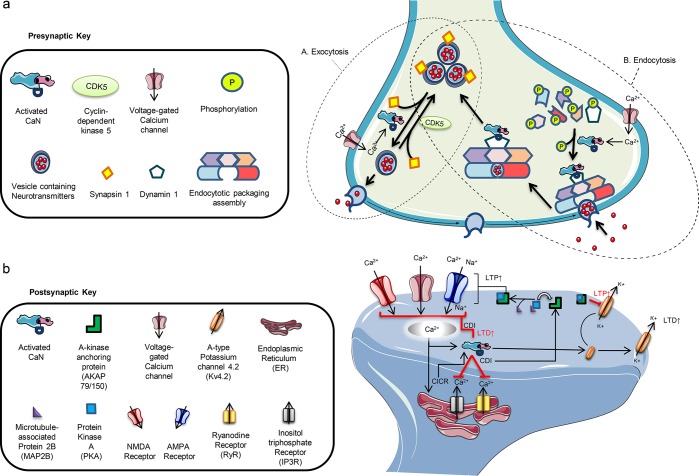
Figure 2.
(a) Presynaptic homeostatic functions of calcineurin (CaN). (A) CaN plays an integral role in vesicle exocytosis by regulating the release in coordination with neurotransmission signaling. The influx of calcium (Ca2+) activates CaN and causes it to dephosphorylate synapsin 1, removing its suppression of vesicles and allowing them to move from the release-reluctant resting pool toward the axonal terminal for release. The process is reversed by the phosphorylation by cyclin-dependent kinase 5 (CDK5). (B) CaN also plays a role in endocytosis of vesicles after activation by Ca2+ and then dephosphorylation of dynamin 1. CaN and dynamin 1 form a complex and move toward the endocytotic packaging assembly where involved proteins allow for the packaging of the vesicle at the axonal terminal, its uptake, and delivery of the vesicle toward the vesicle pool for recycling. This series of synaptic cycling and recycling by CaN is tightly coordinated and coupled to the Ca2+-dependent signaling along with the crosstalk of involved proteins. Interruptions in the processes can disturb the equilibria of neurotransmission and often lead to neuronal impair. (b) Postsynaptic homeostatic functions of calcineurin (CaN). Postsynaptically, CaN tightly regulates several functions. It controls intracellular Ca2+ levels through modulation of voltage-gated calcium channels (VGCCs), NMDA (N-methly-d-aspartate), and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors. Influx of Ca2+ through VGCCs tends to trigger the release of more Ca2+ from the endoplasmic reticulum (calcium-induced calcium release) via ryanodine and inositol triphosphate receptors (RyRs and IP3Rs). The increased Ca2+ load activates CaN, and through a negative feedback control, it dephosphorylates VGCCs, RyRs, and IP3Rs, decreasing the duration of opening and frequency as well as weakening the incoming cationic currents. Ca2+ influx via NMDA receptors (NMDARs) and the Ca2+-permeable AMPA receptors (AMPARs) follows a similar pathway of Ca2+ regulation and decreases long-term potentiation (LTP) through internalization and reduced NMDAR and AMPAR expression and increasing long-term depotentiation (LTD). This Ca2+-dependent inactivation (CDI) reinitiates the opening of these channels by triggering kinases, like protein kinase A (PKA) which complexes with A-kinase anchoring protein 79/150 (AKAP79/150) after guided by microtubule-associated protein 2B (MAP2B), that cause rephosphorylation. In the case of voltage-gated A-type potassium 4.2 (Kv4.2) channels, however, phosphorylation of the channels results in internalization and increased long-term potentiation (LTP) while the process is reversed after dephosphorylation by CaN.