Abstract
The vast majority of functional studies investigating mirror neurons (MNs) explored their properties in relation to hand actions, and very few investigated how MNs responding to mouth actions or communicative gestures. From an anatomical point of view, hand and mouth MNs were recorded in two partially overlapping sectors of the ventral precentral cortex of the macaque monkey: hand MNs were located more dorsally (area F5), mouth MNs more ventrally, extending over the border between the premotor (F5) and the opercular region (DO and PrCO). Despite this anatomical segregation, there is a general assumption that a same neuroanatomical network, having a main source of visual information deriving from the parietal cortex, supports both hand and mouth MNs. In the current review, we challenge this perspective and describe the connectivity pattern of mouth MNs sector, comparing it with the hand MNs sector of F5. The mouth and hand MNs sectors share part of their connectivity pattern, but each also has distinct and specific connections. In particular, the mouth MNs F5/opercular region is connected with premotor, parietal areas mostly related to the somatosensory and motor representation of the face/mouth (area F4, the region between areas F3 and F6, areas PF and SII) and with area PrCO, involved in processing gustatory and somatosensory intraoral input. Unlike hand MNs, mouth MNs do not receive their visual input from parietal regions. Information related to face/communicative behaviors could come from the ventrolateral prefrontal cortex (areas 12 and 46). Further strong connections derive from limbic structures involved in encoding emotional facial expressions and motivational/reward processing. These brain structures include the anterior cingulate cortex, the anterior and mid-dorsal insula, orbitofrontal cortex and the basolateral amygdala. These anatomical data are in agreement with neurophysiological evidence showing that in the mouth MNs F5/opercular region there are neurons responding to facial communicative gestures and also neurons firing during the production of vocalizations. The mirror mechanism is therefore composed and supported by at least two different anatomical pathways: one is concerned with sensorimotor transformation in relation to reaching and hand grasping within the traditional parietal-premotor circuits; the second one is linked to the mouth/face motor control and is connected with limbic structures, involved in communication/emotions and reward processing. This new view of the mirror mechanism provides a new theoretical account to explain different patterns of brain activation in neuroimaging studies and has also important implications for our comprehension of the developmental factors and evolutionary processes involved in mirror neurons origins and functions.
Introduction
The anterior part of the ventral premotor cortex of the monkey has been investigated from anatomical and functional points of view by several researchers. The anatomical work by Matelli and colleagues (1985) showed that the most ventral portion of the agranular frontal cortex, extending from the central sulcus to the inferior limb of the arcuate sulcus, is formed by three different architectonic areas: the primary motor area F1, located in the depth of the anterior bank of the central sulcus and in the convexity immediately rostral to it, and the ventral premotor areas F4 (caudal) and F5 (rostral). In recent years, the combination of the cytoarchitectonic techniques with the neurochemical ones has proven to be useful for providing a more detailed definition of areal borders (see Belmalih et al., 2007). This multi-architectonic approach led to the parcellation of F5 into three sectors: F5c (convexity), F5p (posterior) and F5a (anterior). F5c extends on the convexity of the postarcuate cortex adjacent to the inferior arcuate sulcus, F5p and F5a lie within the postarcuate bank, at different antero-posterior levels (Figure 1A).
Figure 1.
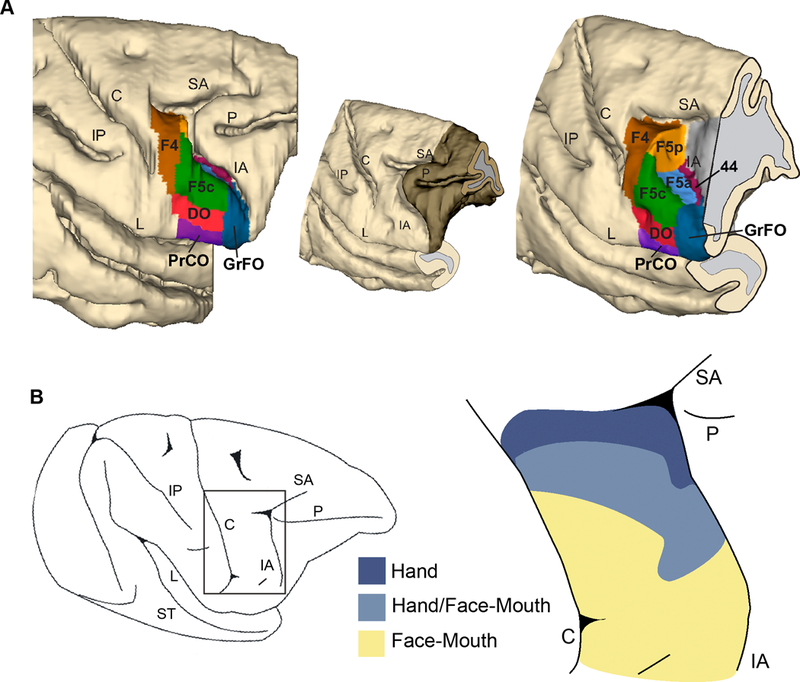
(A). Three-dimensional reconstruction of the frontal lobe of a macaque brain showing the location of the various ventral premotor and opercular areas. The reconstruction is shown from a dorsolateral view (left) and from a rostrolateral (right) view in which the posterior bank of the arcuate sulcus was exposed with dissection of the 3D reconstruction along its fundus. The brain sector removed to expose the postarcuate bank is shown in a darker color in the smaller 3D reconstruction shown in the middle. The map is based on the architectonic features described in Belmalih and colleagues (2009) and in Gerbella and colleagues (2016). (B) Lateral view of the brain of a monkey employed in an experiment aimed to map the functional properties of ventral premotor cortex (left). The rectangle indicates the location of the region enlarged on the right). Cortical fields (right) in which extracellular multi-unit activity is related to brachio-manual (dark blue), mouth (yellow) or brachio-manual and mouth (light blue) effectors. C: central sulcus; IA: inferior arcuate sulcus; IP: intraparietal sulcus; L: lateral fissure; P: principal sulcus; SA: superior arcuate sulcus; ST: Superior temporal sulcus. Modified from Belmalih and colleagues (2009), Gerbella and colleagues (2016) and Maranesi and colleagues (2012).
Several neurophysiological investigations (Kurata and Tanji, 1986; Gentilucci et al., 1988; Rizzolatti et al., 1988; di Pellegrino et al., 1992; Hepp-Reymond et al., 1994; Gallese et al., 1996; Ferrari et al., 2003; Maranesi et al., 2012) showed that area F5 hosts a motor representation of the hand (medially) and of the mouth (laterally) and plays a role in the generation and control of goal-directed motor acts such as grasping or biting (Figure 1B). In addition, other neurons have been described which have visuomotor properties in F5. These neurons fall into two classes: “canonical” neurons and “mirror” neurons. Canonical neurons discharge to the observation of graspable objects and during the execution of a grasping movements (Murata et al., 1997; Raos et al., 2006); mirror neurons (MNs) fire both when the monkey is performing a motor act and also when the same, or a similar act is performed by another individual (di Pellegrino et al., 1992; Gallese et al., 1996; Ferrari et al., 2003; Caggiano et al., 2009; Kraskov et al., 2009; Maranesi et al., 2012, 2015; Bonini et al., 2014; Coudé et al., 2016).
The vast majority of functional studies investigating MNs has explored their properties in relation to hand actions, and was thus focused on the medial part of F5, where the hand is the most represented effector (see Rizzolatti and Luppino, 2001; Rizzolatti et al., 2001, 2014; Borra et al., 2010; Maier et al., 2013; Bonini, 2015). More specifically, neuroanatomical and functional studies have shown that the hand sector of F5 has direct anatomical connections with different hand sectors of the parietal cortex, including areas AIP and PFG (Rozzi et al., 2006; Borra et al., 2008; Bonini et al., 2010; Gerbella et al., 2011). Similar to F5, also these parietal areas host motor neurons coding goal directed motor acts and visuomotor neurons, including MNs in relation to hand actions (Gallese et al., 2002; Fogassi et al., 2005; Rozzi et al., 2008; Maeda et al., 2015). Within the mirror circuit (so called because of the well-established AIP/PFG-F5 connections), it has been proposed that these parietal areas convey visual information regarding hand grasping movements, and are the first hub of the visual input deriving from the temporal cortex, within the STS region. A neuroimaging study in the macaque has further confirmed the idea that information regarding observed hand action is forwarded from the region of the superior temporal sulcus (STS) to area F5 through two different routes passing from the parietal areas AIP and PFG (Nelissen et al., 2011; see Figure 2). Further neurophysiological studies have shown that these parietal-premotor circuits are also essential to support important cognitive functions in relation of action observation such as the understanding of others’ actions and intentions (Fogassi et al., 2005; Bonini et al., 2011).
Figure 2.
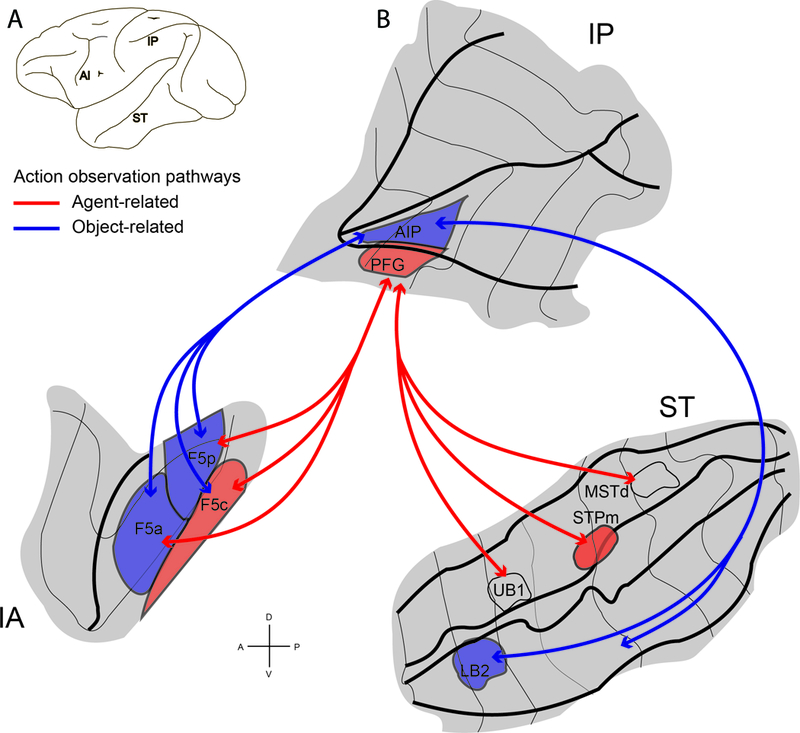
(A) Lateral view of a macaque brain showing the location of three regions involved in action observation: inferior arcuate sulcus (IA), Intraparietal sulcus and inferior parietal lobule (IP), and superior temporal sulcus (ST). (B) Flattened representation of inferior arcuate, intraparietal and superior temporal sulci. Visual information about observed actions can be sent from STS through parietal cortex to premotor area F5 along two functional routes: a STPm–PFG–F5c, agent-related action observation pathway (red lines) and a LB2–AIP–F5a/p object-related action observation pathway (blue lines). The arrows specify the functional routes. Modified from Nelissen et al. (2011).
Other anatomical studies showed that the parietal and premotor mirror areas share numerous other anatomical connections that may extend the functional properties of this neural mechanism beyond the classically described functions (Rizzolatti et al., 2014; Bonini, 2015; Borra et al., 2017). Among these connections, in particular, area F5 is anatomically connected with other cortical motor regions such as F1, F2vr (ventrorostral) and F6 which also are endowed with mirror properties or with properties related to self-other behaviors (Cisek and Kalaska, 2004; Kraskov et al., 2009; Yoshida et al., 2011). In addition, cortico-cortical connections of F5 extend also to areas of the prefrontal cortex (Borra et al., 2011; Gerbella et al., 2011, 2013) where movement-related neurons have been described (Hoshi et al., 1998; Bruni et al., 2015; Simone et al., 2015). This evidence suggests that also a specific sector of the ventrolateral prefrontal cortex (VLPC) is involved in context-based control of actions (either performing with the hand the mouth) and in action observation (Simone et al., 2017).
Despite the fact that monkey mirror neurons have been investigated in several neurophysiological studies, most of these studies were focused exclusively on the visuomotor properties of these neurons in relation to hand actions. The original study by Gallese and colleagues (1996) reported the presence of mirror neurons responding to mouth actions, but the neurophysiological descriptions of the neuronal properties have been limited to hand actions. The work by Ferrari and colleagues (2003) was the first to describe the properties of a new category of MNs responding to mouth actions. The basic properties of visuo-motor congruence described for the hand MNs were also confirmed in that study, but surprisingly, a small percentage of mouth MNs were responding to communicative gestures, such as lipsmacking, a typical macaque affiliative gestures (Figure 3). Mouth MNs were found in the most lateral part of F5 probably extending also over the fronto-opercular region (Ferrari et al., 2003; Maranesi et al., 2012). Further experiments, demonstrated that, within the lateral sector of the PMv, neurons discharging for mouth, hand actions or gestures are often intermingled, thus showing a significant overlap of hand and mouth motor representations (Maranesi et al., 2012). Within this lateral part of the premotor-opercular region, neurons were found that fired when the monkey produced conditioned vocalizations after a prolonged period of training (Coudé et al., 2011).
Figure 3.
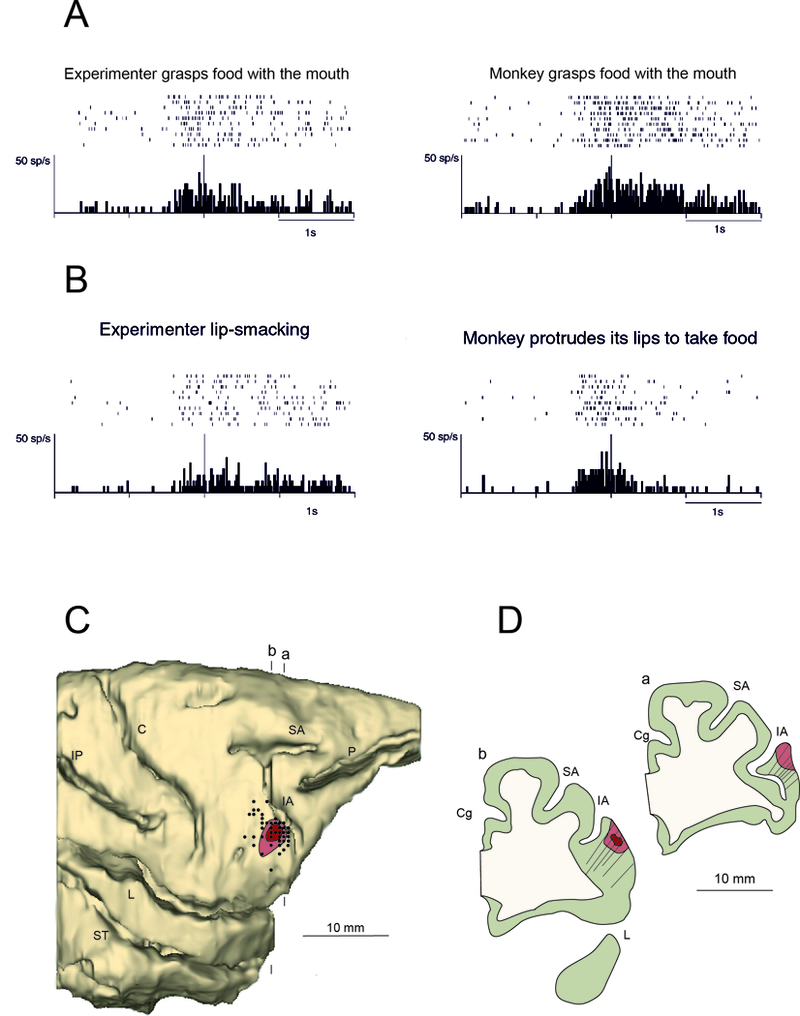
(A) Example of ingestive mouth mirror neuron. Left: the experimenter approaches with his mouth the food held on a stick and grasps it with the teeth; right: the experimenter moves a piece of food to the monkey’s mouth and the monkey grasps it with its teeth and eats it. In each panel, the rasters and the histograms represent the neuron response during a single experimental condition. The histogram represents the average of 10 trials. Rasters and histograms are aligned with the moment in which the mouth of the experimenter (observation conditions) or of the monkey (motor conditions) touched the food. Ordinates, spikes/s; abscissae, time; bin width, 20 ms. (B) Example of communicative mouth mirror neuron responding to lipsmacking. Left: the experimenter makes a lipsmacking action looking at the monkey; right: the experimenter moves a piece of food to the monkey’s mouth; the monkey protrudes its lips and takes the food. During observation of intransitive actions the rasters and histograms alignment were made with the moment in which the action was fully expressed. Conventions as in A. Modified from Ferrari et al., (2003). (C) Lateral view of the 3-D reconstruction of the right hemisphere of the recorded monkey. The dark and light red shaded areas indicate the core and the halo of the injection, respectively. Black dots indicate the penetrations in which mouth mirror neurons were recorded. Vertical lines above and below the brain represent the level of two coronal sections, whose outlines are reported in (D). Abbreviations as in Figure 1. Penetration tracks reported on the outlines of two coronal sections passing through the recorded sector.
Unfortunately, the neurophysiological properties and anatomical properties of this region have received very little attention and therefore there is an assumption that the same neuroanatomical network supporting the hand MNs is also characterizing mouth MNs. There are several considerations and experimental evidence that suggest that this is not the case: 1. From a theoretical point of view it has been suggested that mouth and hand MNs are formed through different developmental trajectories with the former, being involved in communication, relying on face-to-face interactions occurring in the first phases of postnatal life (Casile et al., 2011; Cook et al., 2014; Tramacere and Ferrari, 2016). Hand mirror neurons, instead, are probably formed through the tuning and coupling of motor signals that are visually coupled during infant’s first reaching movement and therefore they could be originally formed to sustain the visual tracking of the own hand. This hypothesis seems to be supported by the neurophysiological finding that some hand MNs respond not only during the observation of others’ actions but modulate neuronal activity during the observation of the own hand grasping (Sakaguchi et al., 2010; Maeda et al., 2015; Maranesi et al., 2015). Such own hand visual feedback modulation seems to be important for self-action monitoring, which is key for infant development of reaching-grasping actions. 2. The lateral part of the ventral premotor cortex, at the border with the opercular region has a different pattern of connectivity from the medial part of F5 where hand MNs have been mostly studied (Matelli et al., 1986; Morecraft et al., 2001; Jürgens, 2002; Simonyan and Jürgens, 2002; Gerbella et al., 2011; Gharbawie et al., 2011). This region of the cortex, for example, projects to the facial nucleus for the innervation of the lower part of the face, likely involved in motor control of the mimic facial muscles (Morecraft et al., 2001). Other anatomical studies showed that the opercular region of the face, has motor representations of the larynx muscles and is anatomically connected with different brainstem nuclei, the putamen, and the reticular formation (Jürgens, 2002; Simonyan and Jürgens, 2002). In addition to the above-mentioned considerations, it should be noted that mouth mirror neurons are mostly located in the ventral sector of PMv, but their presence has also been described in the adjacent opercular region (Ferrari et al., 2003 see Figure 3): the dorsal opercular, area DO, and the granular frontal opercular area, GrFO (Figure 1, Belmalih et al., 2009; Gerbella et al., 2016).
In the current review we are aiming at further investigating the connectivity pattern of the mouth mirror region. We hypothesize that the pattern of connectivity in this region differs from the adjacent and most medial F5 hand cortical sector. In order to test this hypothesis, we first review the pattern of connectivity of the areas belonging to the mouth regions of the premotor and opercular areas (i.e. F5, DO and GrFO) previously published (Gerbella et al., 2016) and compare them with those of the premotor hand region where hand MNs have been found. Second, we present a new case in which we have previously recorded mouth mirror neurons (Ferrari et al., 2003) and tract the anatomical connections of its core region, which was located in between F5, DO and GrFO.
Anatomical connections of the Mouth mirror areas.
Area F5, DO and GrFO share connections with numerous cortical regions and subcortical structures but each of them also has characterizing connections. In this paragraph we will describe the main connectional features of these architectonic areas. In a more recent study (Gerbella et al., 2016) neural tracers were injected in different parts of the monkey frontal opercular regions, including areas DO and GrFO (Figure 4) where mouth MNs were previously found (Ferrari et al., 2003).
Figure 4.
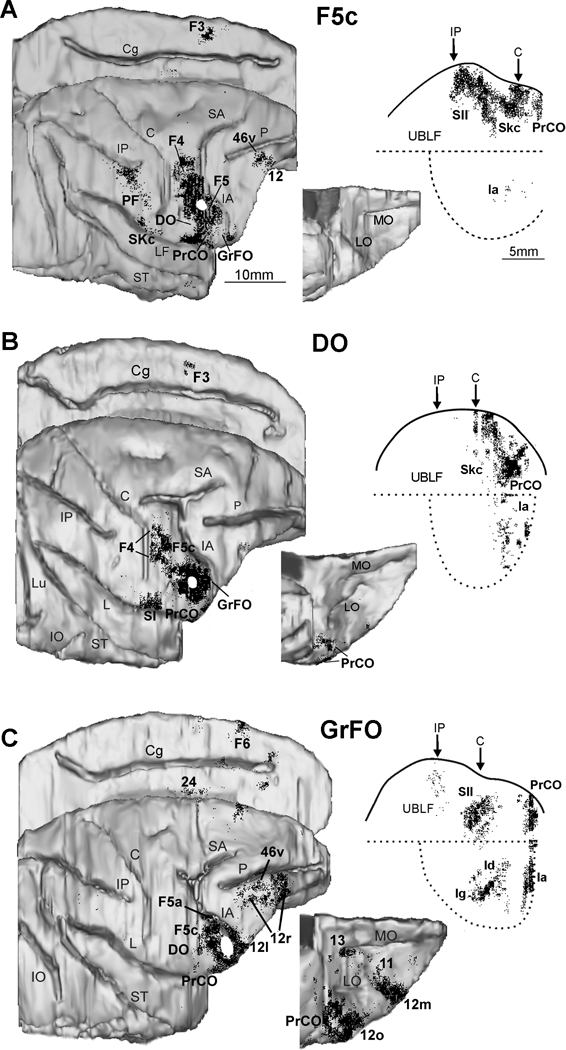
Distribution of the retrograde labeling observed after injections in the ventral part of area F5c (A) and in the opercular areas DO (B) and GrFO (C). The labeling is shown in dorsolateral, medial, and bottom views of the 3D reconstructions of the injected hemispheres, and in 2D reconstructions of the lateral fissure (LF). Each dot corresponds to one labeled neuron. Each 2D reconstruction of the LF was aligned to correspond with the dorsal border of the insula indicated by a straight dotted line; the continuous line marks the lip of the bank, and the curved dotted line marks the border of the insula with the lower bank of the sulcus. Arrows mark the levels of the rostral tip of the intraparietal sulcus (IP) and of the rostralmost level of the central sulcus (C). The location of each tracer injection is shown as a white area on the dorsolateral view of the hemisphere. Scale bar in A applies also to B and C. Cg: cingulate sulcus; IO: inferior occipital sulcus; LO: lateral orbital sulcus; Lu: lunate sulcus; MO: medial orbital sulcus; UBLF: upper bank of the LF; other abbreviations as in Figure 1 (Modified from Gerbella et al. (2011) and Gerbella et al. (2016).
Ventral part of area F5c.
In the agranular frontal cortex, the ventral part of area F5c showed very rich connections with the adjacent part of area F4 (Gerbella et al., 2011), where face and mouth movements are mostly represented (Gentilucci et al., 1988; Huang et al., 1989; Maranesi et al., 2012) (Figure 4a). Weak connections were found with area F3/SMA, mostly in its rostral part, likely in the face representation (Luppino et al., 1991; Mitz and Wise, 1997). Very strong connections involve the frontal opercular areas DO, PrCO, and GrFO and the somatic koniocortex (Gerbella et al., 2011). These regions are known to be involved in gustatory functions, in the sensorimotor control of mouth, pharyngeal, and laryngeal movements (Martin and Sessle, 1993; Ogawa, 1994; Jürgens and Ehrenreich, 2007) and host a sensory representation of the oral cavity (Ogawa et al., 1989). Weaker connections were found with the prefrontal cortex, and specifically with the intermediate part of area 12r (Gerbella et al., 2011) involved in the contextual control of hand and mouth actions (Simone et al., 2015) and in action observation (Nelissen et al., 2005; Simone et al., 2017). In the parietal cortex, strong connections involve the upper bank of the lateral sulcus, at the level of the face/mouth representation of the second somatosensory cortex, SII (Krubitzer et al., 1995; Fitzgerald et al., 2004). In the IPL convexity cortex, strong connections are present in the face-related area PF (Rozzi et al., 2008) and in area 2, where the face is mostly represented (Pons et al. 1985), but they are virtually absent in PFG and AIP. These data are in line with many electrophysiological investigations indicating a specific role of this premotor sector in mouth/face motor control (Maranesi et al., 2012).
Area DO.
Area DO is strongly connected with the ventral part of area F5c and shows a similar connectivity pattern (Gerbella et al., 2014) (Figure 4b). In fact, it is connected with the ventral part of area F4, areas PrCO and GrFO, and the somatic koniocortex (Skc). However, differently from F5c, it is strongly connected to the agranular part of the insula and lacks the connections with the parietal and the prefrontal cortex. These data suggest that, similarly to the ventral part of area F5c, area DO is involved in the motor control of mouth, especially by integrating viscero-motor information coming from the insular region.
Area GrFO.
Area GrFO has its strongest connections with the adjacent opercular areas DO and PrCO (Gerbella et al., 2016) (Figure 4c). In the ventrolateral prefrontal cortex, area GrFO has also very strong connections with area 12l and with the hand and mouth-related fields of areas 46v and 12r. In the agranular premotor cortex, the region mostly connected to GRFO is the hand-related ventral premotor area F5a. Other frontal connections involve the ventral part of area F5c and the caudal part of area F6/pre-SMA. In the parietal cortex, the only region strongly connected is SII, at the level of its face and hand representations. In contrast with areas F5c and DO, area GrFO shows rich connectivity with several components of the limbic system, including orbitofrontal areas 12o, 12m, and 11, the agranular and dysgranular insula, the agranular cingulate area 24, and the amygdala.
Based on these data, it has been proposed that GrFO could represent a gateway for the access of limbic information, about the subjective value and the emotional significance of external stimuli or about internal states, to the premotor areas involved in selecting appropriate hand and mouth/face actions (Gerbella et al., 2016).
All these areas show their strongest connections with the ventral motor and frontal opercular cortex. In particular, they are strongly interconnected and receive strong projection from mouth/face-related motor fields (Cipolloni and Pandya, 1999; Gerbella et al., 2011, 2014). However, F5c and GrFo has stronger connections with hand related motor fields (F5p, F3/F6 and 24c) than PrCO, while area DO almost completely lacks these connections. This is in line with functional evidence showing the presence of a very large representation motor face and mouth representation over the whole region (Gentilucci et al., 1988; Godschalk et al., 1995; Maranesi et al., 2012), while hand grasping responses mostly involve the dorsal part of PMv extending ventrally toward the opercular margin, in the location of area GrFO (Nelissen and Vanduffel, 2011).
In the described neural tracing experiments the authors targeted the core of architectonically defined areas but, as pointed out above, the neurons endowed with mouth-related motor and mirror properties are located in a wide region, extending over different architectornic areas (Ferrari et al., 2003; Maranesi et al., 2012). To this day, no anatomical studies specifically tracked the connections of the functional sector hosting mouth-mirror neurons. To fill this gap of knowledge, we functionally identified the cortical sector hosting mouth mirror neurons and injected neural tracers in the core of this region in order to assess its specific pattern of cortical and subcortical connections.
Analysis of a new case in which mouth MNs were recorded
Figure 5 shows the reconstruction of the injection site and the location of penetrations (see details of methods in legend of Figure 5) in which mouth mirror neurons have been recorded (see also Ferrari et al., 2003). These penetrations extend over the location of the ventral part of area F5c, area DO and GrFO. The large majority of mouth mirror neurons were found in the cortical convexity (more than 85% at a depth ranging from 200 to 1500 mm). Accordingly, the injection site is located in the cortical convexity, close to the crown of the inferior arcuate sulcus (Figure 5), in the ventral third of its extension, in a location very rich in mouth mirror neurons. In order to illustrate the properties of MNs in this regions, the discharge of one mouth related communicative mirror neuron recorded in this region is shown in Figure 3.
Figure 5.
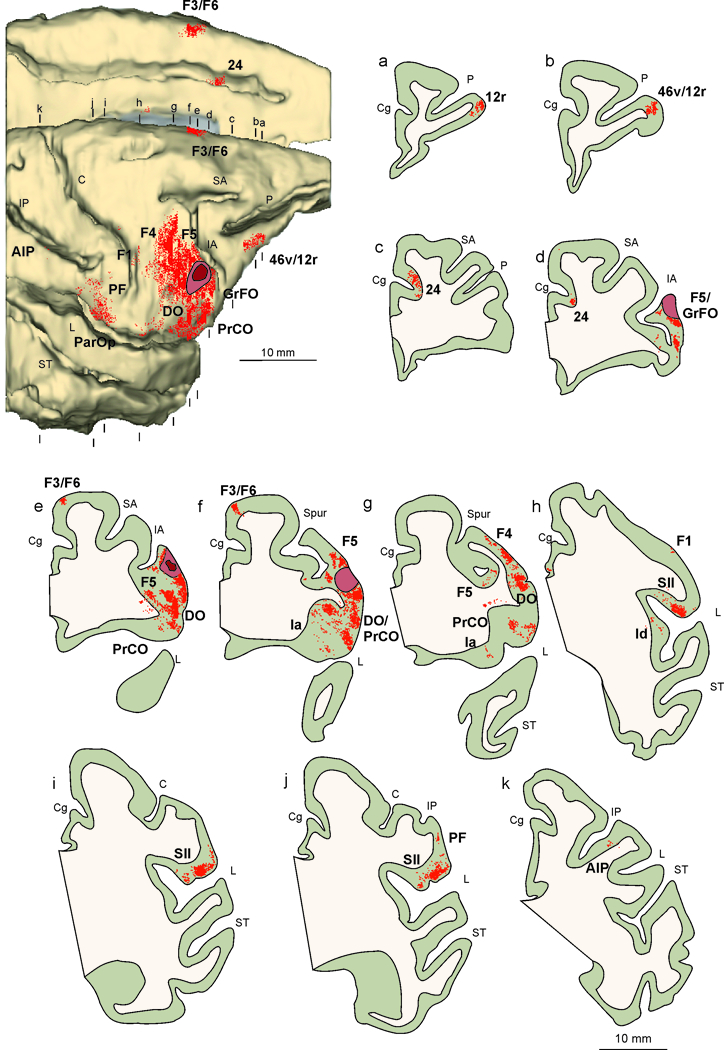
Distribution of the retrograde labeling observed after injection of neural tracer WGA-HRP in the functionally identified mouth mirror sector, shown in dorsolateral and mesial views of the 3D reconstructions (upper-left), and in drawings of coronal sections arranged in a rostral to caudal order (a–k). Abbreviations and conventions as in Figures 1 and 4.
-Methods description. The experiment was carried out on an adult macaque monkey (Macaca nemestrina), previously employed in electrophysiological studies of mouth mirror neurons (Ferrari et al., 2003). The animal handling, as well as the surgical and experimental procedures complied with the European law on the humane care and use of laboratory animals (directive 2010/63/EU), they were authorized by the Italian Ministry of Health (D.M. 294/2012-C, 11/12/2012) and approved by the Veterinarian Animal Care and Use Committee of the University of Parma (Prot. 78/12 17/07/2012).
-Electrophysiological recordings and choice of the injections location. Neuronal activity was recorded by means of single tungsten microelectrodes (impedance 0.5–1.5 MΩ, measured at 1 kHz) inserted through the intact dura. Neuronal activity was amplified and monitored on an oscilloscope. Individual action potentials were isolated with a dual voltage-time window discriminator (Bak Electronics, Germantown MD, USA). The output signal from the voltage-time discriminator was monitored and fed to a PC for analysis (for a complete description of the methodology employed, see Ferrari et al., 2003). The regions to be injected were selected on the basis on the results obtained during the electrophysiological experiments carried out in the same animal. In particular, we targeted the core of the specific sector where mouth mirror neurons were recorded (Figure 3C-D).
-Tracers injections and histological procedures. The neural tracer wheat germ agglutinin-horseradish peroxidase conjugated (WGA-HRP, 4% in distilled water, SIGMA, St. Louis, Missouri) was injected through the intact dura at specific coordinates of the recording grid. A recording session performed immediately before the tracer injection was carried out in order to confirm the presence of reliable mouth mirror neuron activity and functional properties coherent with those expected based on the previous electrophysiological experiments. Tracers were slowly pressure injected through a Hamilton microsyringe (Reno, NV). About 1 week before sacrificing the animal, electrolytic lesions (10 μA cathodic pulses per 10 s) were performed at known coordinates at the external borders of the recorded region. After the appropriate survival period for tracer transport (48 hours), the animal was deeply anesthetized with an overdose of sodium thiopental and perfused through the left cardiac ventricle with saline, 3.5% paraformaldehyde and 5% glycerol in this order, prepared in phosphate buffer 0.1 M, pH 7.4. Each brain was then blocked coronally on a stereotaxic apparatus, removed from the skull, photographed, and placed in 10% buffered glycerol for 3 days, followed by 20% buffered glycerol for 4 days. Finally, each brain was cut frozen into coronal sections of 60μm thickness. One section of each five was processed for WGA-HRP histochemistry with tetramethylbenzidine as chromogen (Mesulam, 1982); each second and fifth section of a series of 5 was stained using the Nissl method (thionin, 0.1% in 0.1 M acetate buffer, pH 3.7), for the reconstruction of electrodes tracks.
-Reconstruction of the injection sites, identification of the recorded region, distribution of labeled neurons The criteria used to histologically identify the injection sites and to recognize the neural tracers labeling have been described in previous studies (Luppino et al., 2003; Rozzi et al., 2006). The injection site presented in this study is completely confined within the cortical grey matter. Its location was reported on a three-dimensional (3D) reconstruction of the injected hemisphere. The penetrations grid was reconstructed based on electrolytic lesions, stereotaxic coordinates, penetrations depth and the observed functional properties, and finally superimposed onto the anatomical reconstruction (Figure 3 and 5). The distribution of retrograde cortical labeling was plotted in sections spaced 600 μm apart from each other, together with the outer and inner cortical borders, using a computer based charting system. Data from individual sections were also imported into dedicated software (Bettio et al., 2001) allowing us to create 3D reconstruction of the hemispheres from individual histological sections containing labeled cells. The result of this processing provides a realistic visualization of the labeling distribution for a precise comparison of data relative to different hemispheres. The criteria and maps adopted for the areal attribution of the labeling were the same to those adopted in previous studies (Rozzi et al., 2006; Borra et al., 2008; Gerbella et al., 2011). Specifically, in the IPL, the gyral convexity areas were defined according to Gregoriou et al. (2006) and those of the lateral bank of the intraparietal sulcus according to Borra et al. (2008). The labeling in the dysgranular and agranular frontal, opercular frontal and rostral cingulate areas was attributed in accordance with Walker (1940), Matelli et al. (1985, 1991) and Belmalih et al. (2009). The prefrontal cortex was subdivided according to Carmichael and Price (1995). We plotted amygdalar, striatal and thalamic labeled cells in sections spaced 300 μm apart together with the outline of the ventricles and of blood vessels, using the aforementioned computer based charting system. Borders of amygdalar and thalamic nuclei, defined in adjacent Nissl-stained sections, were then superimposed on the plots of labeled cells using the outline of the ventricles and of blood vessels, with the aid of a microprojector and a camera lucida (see Matelli et al., 1989; Contini et al., 2010; Gerbella et al., 2014). The borders of the thalamic nuclei were defined according to the cytoarchitectonic criteria and the nomenclature used by Olszewski (1952), except for nucleus ventralis lateralis defined according to previously described cytoarchitectonic criteria (Matelli et al., 1989; Matelli and Luppino, 1996). The amygdalar complex was subdivided according to the criteria described by Amaral et al. (2003).
Results of the Cortical connections of the mouth MNs sector
The distribution of labeled cells after WGA-HRP injection in the mouth mirror sector is shown in Figure 5. Rich labeling was observed in a wide region extending over the ventral premotor areas F5 and F4. The labeled territory mainly involves the ventralmost part of these areas, at the level of the motor representation of face and mouth (Gentilucci et al., 1988; Godschalk et al., 1995; Ferrari et al., 2003; Maranesi et al., 2012). In this region there are also neurons activate by somatosensory stimulation of the face and the mouth (Gentilucci et al., 1988; Maranesi et al., 2012) and by the observation of others’ actions, performed with the hand, the mouth or even a tool (Ferrari et al., 2003, 2005; Maranesi et al., 2012). The labeled territory slightly extends more dorsally in the purely hand representation of area F5. Strong labeling was also observed more ventrally in the cortical convexity and in the frontal operculum (areas DO,and PrCO), including also the “laryngeal motor cortex” described by Simonyan and Jürgens (2003). Further labeled cells in the premotor cortex have also been observed in the mesial cortex at the border between area F3 and F6, corresponding to the level of the motor representation of the mouth (Luppino et al., 1991) and in the cingulate area 24, in a location compatible with the face/mouth motor representation (Morecraft et al., 2007). A few labeled cells are also present more caudally in the primary motor cortex (F1). Beyond the frontal lobe, labeled neurons were observed in the anterior and mid-dorsal part of the insula, whose stimulation is known to evoke disgust-related behaviors and affiliative facial expressions, respectively (Caruana et al., 2011; Jezzini et al., 2012). Further labeling was observed in somatosensory face/hand fields the parietal operculum (area PFop and secondary somatosensory cortex – SII) and in the rostral part of inferior parietal lobule (mainly area PF). Connections have also been observed with the ventral part of the ventrolateral prefrontal cortex (VLPF, areas 46v and 12r), in a sector compatible with the one where hand and mouth movement related activity has been recorded (Simone et al., 2015).
Results of the Subcortical connections of the mouth mirror neuron sector
Figure 6A shows the distribution of anterograde labeling at different rostro-caudal levels of the striatum. The labeling in the putamen extends in the rostrocaudal direction from about 3–4 mm caudal to about 2–3 mm rostral to the level of the anterior commissure (AC). The labeled region posterior to the AC corresponds to the ventral sector of the motor putamen hosting mouth and face representations (Alexander and DeLong, 1985). The labeled region of the putamen, anterior to the AC, was recently defined as involved in foraging behavior and part of circuits related to motivated behaviors (Tremblay et al., 2015). Finally, some projections were located in a very lateral part of the caudate, close to the internal capsule, extending in rostrocaudal direction from about 1 mm caudal to about 2–3 mm rostral to the level of the AC, in a region that could be involved in selection and preparation of motor behavior as recently proposed by Tremblay and colleagues (2015).
Figure 6.
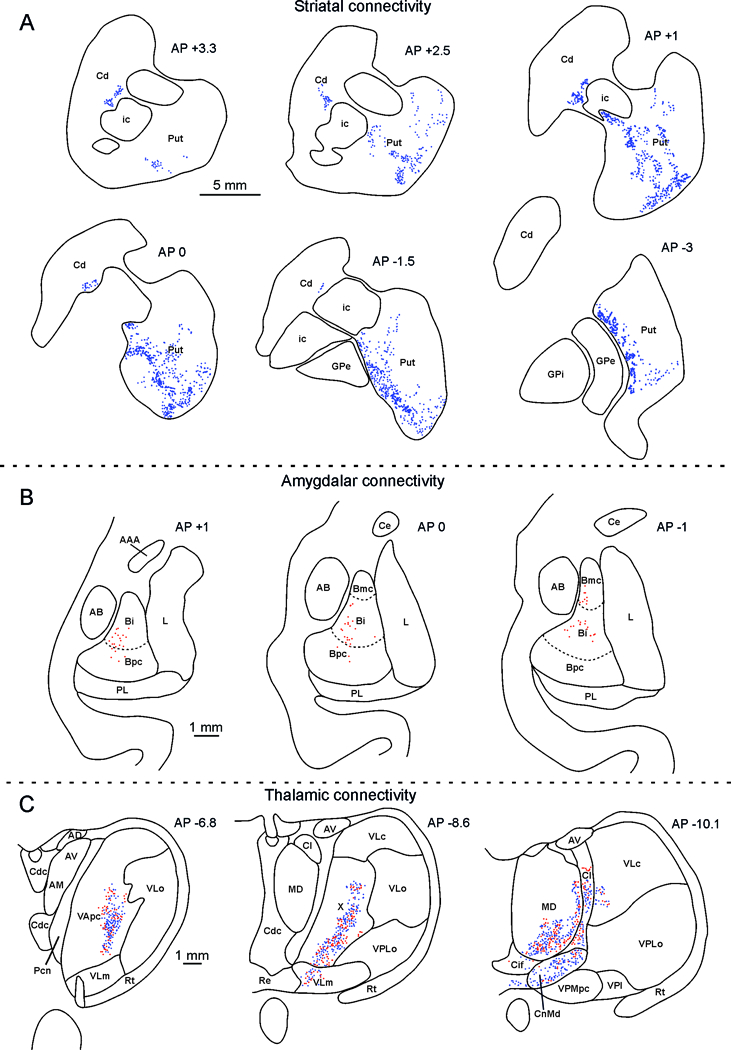
Striatal, amygdala and thalamus distribution of the labeling observed after Mk1 injection. (A). Drawings of coronal sections through the striatum showing the distribution of the anterograde labeling; the dot density is proportional to the density of the observed labeled terminals (one dot is equivalent to about 15–25 labeled terminals). The sections are shown in a rostral to caudal order and their AP level is indicated in terms of distance in mm from the anterior commissure (AC). Scale bar applies to all sections. Cd: caudate nucleus; GPe: external globus pallidus; GPi: internal globus pallidus; ic: internal capsule; Put: putamen. (B). Distribution of retrogradely labeled amygdalar neurons. Each dot corresponds to one labeled neuron. For each case, the labeling is shown in two drawings of coronal sections, selected at different AP levels. The dashed lines mark the borders of the magnocellular, intermediate, and parvocellular subdivisions of the basal nucleus. Scale bar applies to all sections. AAA: anterior amygdaloid area; AB: accessory basal nucleus; Bmc: magnocellular subdivision of the basal nucleus; Bi: intermediate subdivision of the basal nucleus; Bpc : parvocellular subdivision of the basal nucleus; Ce: central nucleus.L: lateral nucleus; PL: paralaminar nucleus. (C). Distribution of labeled thalamic neurons and terminals. The labeling is shown in drawings of coronal sections in rostral to caudal order selected at different AP levels according to the atlas of Olszewski (1952). Each red dot corresponds to a single labeled neuron, each blue dot is equivalent to about 15–25 labeled terminals. Scale bar applies to all sections. AM anterior medial nucleus, AV anterior ventral nucleus, Cl central lateral nucleus, Cn.Md centromedian nucleus, Csl central superior lateral nucleus, LD lateral dorsal nucleus, MD mediodorsal nucleus, MDmc mediodorsal nucleus, magnocellular part, MDmf mediodorsal nucleus, multiform part, MDpc mediodorsal nucleus, parvicellular part, Pcn paracentral nucleus, Pf parafascicular nucleus, Re reuniens nucleus, THI habenulointerpeduncular tract, TMT mammillothalamic tract, VAmc ventral anterior nucleus magnocellular part, VApc ventral anterior nucleus, parvicellular part, VLm ventral lateral nucleus, medial part, VLo ventral lateral nucleus, oral part, VPM ventral posterior medial nucleus, VPMpc ventral posterior medial nucleus, parvicellular part, X nucleus X of Olszewski (1952).
Figure 6B shows the distribution of retrograde labeling at different rostro-caudal levels of the amygdala. Virtually all labeled cells are located in the basal nucleus. Noteworthy, this nucleus is the major target of inferotemporal projections and hosts neurons coding face identity and expressions (e.g., Gothard et al., 2007) or gaze directions (Tazumi et al., 2010), and neurons involved in detection of direct gaze (Tazumi et al., 2010), or activated when looking at the eyes of conspecifics (Mosher et al., 2014). Note that no anterograde labeling was found, similar to what occurs after injections in the architectonically defined area GrFO (Gerbella et al., 2016). These results confirm the notion that the ventral premotor/frontal opercular region receives but does not send projections to the amygdala.
Figure 6C show the distribution of retrograde labeling at different rostro-caudal levels of the thalamus. In the rostral part of the thalamus labeled cells were observed in the nuclei associated with sensory-motor functions, such as the ventral anterior nucleus (VA) and area X. The observed labeling extends posteriorly in the mediodorsal nucleus (MD), and in the intralaminar central lateral and median nuclei (Cl and Cn.Md). Note that the labeled sector of MD (MDmc) is known to be connected with the prefrontal areas 46v and 12, which are, in turn, connected with the injected region.
Two different anatomo-functional pathways supporting hand and mouth mirror neurons
The objective of this manuscript was to assess the hypothesis that the region containing mouth MNs and corresponding, not only to the lateral part of F5c, but also encompassing the cytoarchitectonic areas GrFO and DO, have a pattern of connectivity different from the one described for the hand motor regions of the PMv, which contains hand MNs. To this end, we first reviewed the anatomical literature describing the pattern of connectivity of the lateral part of F5 and of the frontal opercular region (F5/opercular) in which MNs have been found. We then presented a new tract-tracing experiment. In this experiment we injected a neural tracer in the region in which mouth MNs have been previously recorded. The results of this experiment were in agreement with the evidence from previous cases (see Figure 4) in which the injections involved the architectonically defined areas F5c, GrFO, and DO. Specifically, the mouth MNs F5/opercular region showed connections with premotor, cingulate and parietal territories mostly related to face and mouth movements, such as the ventral part of area F4, the region between areas F3 and F6, and anterior parietal area PF. Connections were also observed in the anterior and mid-dorsal part of the insula, whose electrical stimulation is known to evoke disgust-related behaviors and affiliative facial expressions, respectively (Caruana et al., 2011; Jezzini et al., 2012). Other connections were found in area PrCO, known to be involved in gustatory functions and hosting a sensory representation of the oral cavity (Ogawa et al., 1989).
It is important to note that the mouth and hand sectors of the ventral premotor cortex indeed share part of the connections. In fact they both target the mesial premotor cortex (F3/F6), the ventral lateral prefrontal cortex (areas 12 and 46), SII, the inferior parietal cortex and the insula. However, the sectors of targeted areas are adjacent to each other, resembling the somatotopic organization very likely supporting sensorimotor transformation (Rizzolatti and Luppino, 2001; Rizzolatti et al., 2014). For example, the IPL connections of the mouth and hand premotor sectors target the PF and PFG regions, respectively (see below for further discussion). In addition, some of the injections made at the border between hand and mouth F5 region (Gerbella et al., 2011) show a partial anatomical and functional overlap. This suggests that some aspects of the motor control of the hand and the mouth must be neurophysiologically coordinated in order to support synergies during hand-mouth interaction when the monkey grasps food and brings it to the mouth, for instance (Ferrari et al., 2003). In this hand-mouth overlap premotor region, in fact, some neurons firing for both hand grasping and mouth actions have been described (Gentilucci et al., 1988; Ferrari et al., 2003; Maranesi et al., 2012).
Despite this shared pattern of connectivity, present data show that the mouth F5/opercular territory has also specific anatomical connections, distinguishing it from the medial part of F5, where hand MNs were found. Specifically, the strong connections that the hand MNs F5 showed with the parietal regions of AIP and PFG, considered as the main source of visual input to premotor hand MNs (Rozzi et al., 2006; Borra et al., 2008; Bonini et al., 2010; Gerbella et al., 2011; Nelissen et al., 2011), are very weak or virtually absent after injections in the mouth MNs region. From this parietal region, only area PF has significant connections with the mouth MNs region (Gerbella et al., 2011, 2016). PF has been shown to be a somatomotor area rich of neurons with properties linked to mouth actions, but lacks MNs and, more generally, is devoid of visual responses (Hyvärinen, 1981; Rozzi et al., 2008). Thus, the visual input related to other faces during actions/gestures could reach the F5/Opercular sector through other pathways not involving the parietal cortex. Based on anatomical and functional findings, we propose that one of these pathways may involve the prefrontal cortex (see also Bonini, 2015; Rozzi and Coudé, 2015). In fact, the ventrolateral prefrontal cortex (VLPC) is connected both with temporal sectors participating to the visual coding of biological motion (Barbas, 1988; Petrides and Pandya, 2002; Gerbella et al., 2010, 2013; Borra et al., 2011) and, as demonstrated in the present study, with the mouth MNs sector. In addition, VLPC contains neurons responding to visual stimuli of faces and of facial communicative gestures (Ó Scalaidhe et al., 1997, 1999; Sugihara et al., 2006; Romanski and Diehl, 2011; Kuraoka et al., 2015). It is also likely that information related to facial expressions and/or its emotional content could reach the F5/opercular region through other pathways involving the orbitofrontal, insula and the amygdala (see below for further elaboration).
The functional specificity of hand and mouth premotor regions is further supported by the presence of different patterns of subcortical projections. The mouth MNs region projects to two sectors of the putamen. The caudal one is involved in movement execution, and corresponds to the ventral sector of the motor putamen where face/mouth movements are represented (Alexander and DeLong, 1985). The rostral one is known to be involved in in the selection and preparation of movements (Schultz and Romo, 1992; Gerardin et al., 2004; Tremblay et al., 2015). It is also possible that some of the labels here reported and located in the rostral part of the putamen, involve a sector described by Tremblay and colleagues (2015) as playing a crucial role in integrating motivational behavior with specific motor programs related to the mouth and in foraging behaviors. This subsector could be therefore part of the limbic network as suggested by Haber (2006).
The mouth mirror sector is also connected with the thalamus. Thalamic projections derive from the anterior nuclei associated with sensory-motor functions (VA; X), but also from more posterior nuclei such as MD. This nucleus is also connected with the prefrontal areas 46v and 12, which contain neurons responsive during the execution of hand and mouth actions (Simone et al., 2015) and during action observation (Simone et al., 2017), in turn linked with the mouth mirror sector. This trans-thalamic interplay could modulate the efficacy of direct inputs from one cortical area to another (see Sherman, 2007; Saalmann and Kastner, 2011). This interplay could likely reflect the integration of hand and mouth motor synergies in coordinated motor sequences that are required during foraging behaviors.
There are additional significant differences between the connections of F5/opercular region, where mouth MNs have been found, and those located more dorsally in the hand MNs region of F5. First, while the hand area of F5 projects to sectors of mesencephalic, pontine and bulbar reticular formation, which are sources of spinal cord projections for the motor control of the hand (Kunzle, 1978; Gerbella et al., 2010) the mouth sector of PMv/opercular projects to different mesencephalic nuclei (Solitary tract, trigeminal and facial nuclei) and the pontine gray matter, which are involved in coordinating facial muscles with larynx and respiratory motor centers, either during facial expressions and/or utterances (Larson, 1991; Morecraft et al., 2001; Simonyan and Jürgens, 2003; Jürgens, 2009). Second, differently from the hand regions, the F5/opercular mouth region has strong connections with the anterior cingulate cortex, the anterior insula, and the basolateral amygdala. The projections to the anterior cingulate cortex are likely targeting a region involved in emotional processing of information in relation to reward value or to the relevance of behaviors linked to outcomes (Hayden et al., 2010; Cai and Padoa-Schioppa, 2012; Chang et al., 2013). Interestingly, neuroimaging studies showed that the observation and imitation of facial expressions of emotions activates a region of the anterior cingulate cortex (Carr et al., 2003; Singer et al., 2004). Recently, it has been described in a human patient that the electrical stimulation of the insula produces facial expressions of laughing, and the intracranial electroencephalogram recording from the same electrode sites show that there was specific activity correlated with the observation of laugh (Caruana et al., 2016). The link of the mouth region of the F5/opercular region to the anterior cingulate cortex could be therefore important to process information regarding the food value for programming and selecting ingestive activities with the mouth, but that may also be exploited within the domain of social communication to couple specific values to behavioral patterns expressed during social interactions and facial expressions. These two alternatives are not necessarily excluding each other.
It is worth noting that the cingulate cortex connected with the mouth mirror region is also involved in mouth motor control and in vocal communication as demonstrated by previous neurophysiological recordings in monkeys conditioned to produce vocal calls (West and Larson, 1995). The possible involvement of ACC-F5/opercular network in the control of vocalizations is further supported by studies showing the presence of neurons responding to the production of volitional calls in the lateral sector of F5/DO region (Coudé et al., 2011; Hage and Nieder, 2013) and of the elicitation of larynx movement in monkeys and laughter in humans during electrical stimulation (Hast et al., 1974; Simonyan and Jürgens, 2003; Coudé et al., 2011; Caruana et al., 2016).
The specific patterns of neuroanatomical connections with the insular cortex are different between the hand and the mouth fields here investigated. Our and previous findings show that the lateral sector of F5/opercular region is connected with the anterior insula, within a region that is not only involved in gustatory processing, but also in the production of facial expression (Caruana et al., 2011; Jezzini et al., 2015). In fact, electrical stimulation of this region of the monkey insula evokes the production of facial expressions of disgust and, in some cases, of lipsmacking (Caruana et al. 2009). A recent anatomical study (Jezzini et al., 2015) showed that this anterior insular sector could be considered as a part of the limbic system and is involved in integrating sensory/visceral inputs with appropriate motor responses (Caruana and Gallese, 2012; Jezzini et al., 2015). In agreement with these monkey investigations, several neuroimaging studies in humans have demonstrated that the anterior insula is part of the mirror system for the decoding of emotions. In fact, there is evidence that this anterior sector is activated both when subjects observe (or experience) facial expressions of emotions and produce the same motor facial pattern involved in that specific emotions (Carr et al., 2003; Wicker et al., 2003; Singer et al., 2004).
The projections from the basolateral amygdala further supports the involvement of the F5/opercular region within the limbic circuit, in which information regarding the valence of stimuli, in relation to face processing is decoded. Several studies have showed that neurons in basolateral amygdala respond to faces and to facial expressions of emotions (Adolphs et al., 1994; Fried et al., 1997; Gothard et al., 2007; Liu et al., 2016; Minxha et al., 2017) The basolateral amygdala has been shown also to be responsive to tasks were social judgments and valence of stimulus (social or not social) have to be made (Chang et al., 2015). It is interesting to note that neuroanatomical studies also found that this region of the amygdala is connected with the orbitofrontal cortex (areas 12 and 13; Carmichael and Price, 1995), which also receives projections from the F5/opercular sector. This suggests that the mouth field of the F5/opercular sector is part of a network that could be involved in processing the subjective value of rewards (Padoa-Schioppa and Assad 2008). Indeed such coding is key for behavioral choices that must weigh the costs of specific activities, in terms of economical choices. However, such process could extend to social contexts, as recently demonstrated in a single neuron study (Azzi et al., 2012).
Conclusions
The findings described here overall indicate that MNs are present in different cytoarchitectonic areas and that their specific properties are linked not only to the type of effector involved (hand or mouth) but also to different anatomical pathways. In fact we provided evidence that mouth MNs are present in the later part of F5c and the frontal opercular region, which have different anatomical connections from the medial sector of F5c where hand MNs have been described. One of the most striking finding emerging from our analysis is that, while the hand MNs are part of parietal-premotor networks clearly involved in visuomotor transformation for reaching-grasping, the mouth MNs sector shares only part of this network, lacking the rich visual connections of AIP and PFG regions (Figure 7). These parietal areas, in fact, contain hand MNs as well, and receive their visual input from the STS region (Nelissen et al., 2011). Given such differential anatomical pattern of connectivity between the hand and mouth MNs sectors of F5/operculum, it follows that information about the vision of others’ face-mouth actions must reach the mouth mirror sector through different paths. We propose that these paths may involve the ventral prefrontal cortex, the insula and the amygdala.
Figure 7.
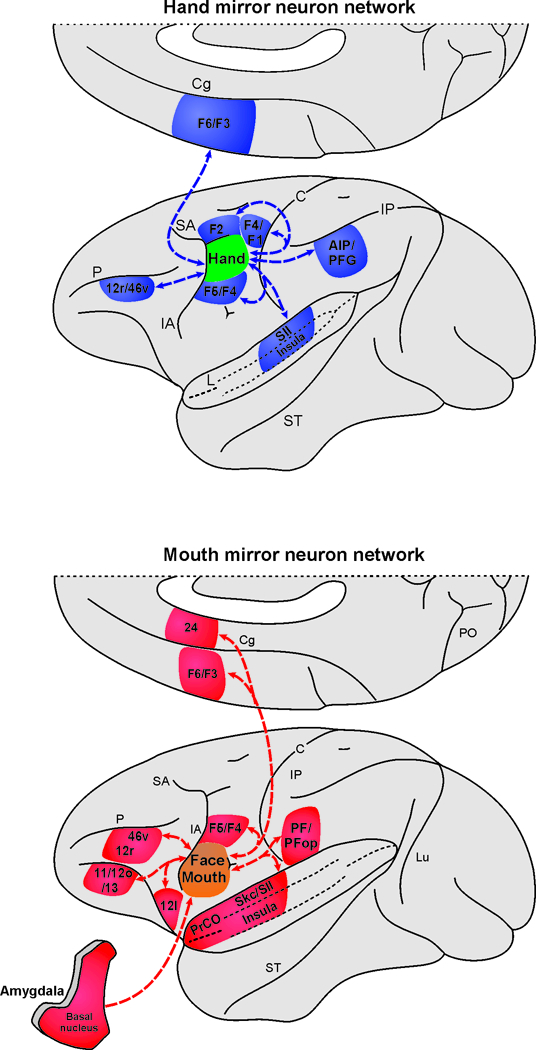
Summary of the hand and the mouth mirror neuron networks. The connectivity of the hand mirror neuron network is based on the description of neural tracer injections placed in the dorsal part of area F5 in which the hand mirror neurons were found. Specifically, the connections indicated are based on those observed in previous works (Matelli et al., 1986; Bonini et al., 2010; Gerbella et al., 2011). The connectivity of the mouth mirror neuron network is based on the description of neural tracer injections placed in the ventral part of area F5 and in the opercular areas GrFO and DO in which the mouth mirror neurons were found and that is described in this study.
Another important differentiation between the hand and mouth MNs sectors is that the latter has specific connections with brain regions that are part of the limbic system and involved in emotion/reward processing. Despite several studies in humans and monkeys have suggested the possibility of a mirror mechanism in humans for emotions (Carr et al., 2003; Wicker et al., 2003; Caruana et al., 2016; see also Rizzolatti and Sinigaglia, 2016), scholars simply extended the notion of a matching mechanism from the hand to the face, without considering that the differential pattern of activation found between hand actions and facial expressions rely on different anatomical pathways and therefore imply that they likely accomplish different functions. This idea is not new. In fact, previous theoretical works (Del Giudice et al., 2009; Casile et al., 2011; Ferrari et al., 2013; Cook et al., 2014; Tramacere and Ferrari, 2016) have analyzed how visual and motor information could possibly become matched in the course of development. There is ample convergence on the hypothesis that visuomotor associations for hand actions emerge during development, when infants launch their first attempts to reach and grasp objects: the motor commands controlling the hand are coupled with the visual feedback of their own hand. Thus, the capacity of visually track the arm movement in space is central for the development of the coupling of visuomotor information within the parietal-premotor circuits (Del Giudice et al., 2009; Casile et al., 2011; Ferrari et al., 2013; Cook et al., 2014; Tramacere and Ferrari, 2016). In contrast, mouth MNs must rely on a different network maturational process that is highly canalized during development (Ferrari et al., 2013) and relies on the interaction between the infant and the caregiver in the early phases of development. Infants, in fact, cannot see their own face, however their mother/caregiver provide continuous feedback to their facial expressions and emotional state, often imitating them and thus reinforcing the forms and the emotional content of their behavior (Murray et al., 2016). Mouth MNs could therefore be formed within a context of communication and emotional exchange. It is important to note, however, that part of the mouth MNs network can still maintain an original link with the hand MNS network, since hand-mouth motor synergies are extremely important in infant development for feeding behaviors. In fact, neurophysiological work on the monkey show the presence, in the lateral sector of F5c, of MNs motor and visual responses both to hand and mouth actions, or actions related to bringing food to the mouth (Ferrari et al., 2003; Maranesi et al., 2012).
This new view of the mirror mechanism provides a new theoretical account to explain different patterns of brain activation in neuroimaging studies and has also important implications for our comprehension of the developmental factors and evolutionary processes involved in mirror neurons origins and functions.
ACKNOWLEDGEMENTS
This work was supported by the Division of Intramural Research, NICHD, and NIH P01HD064653.
References
- Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR (1985) Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, Prather M (2003) The amygdala: Is it an essential component of the neural network for social cognition? Neuropsychologia 41:235–240. [DOI] [PubMed] [Google Scholar]
- Azzi JCB, Sirigu a, Duhamel J-R (2012) Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci 109:2126–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H (1988) Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol 276:313–342. [DOI] [PubMed] [Google Scholar]
- Belmalih A, Borra E, Contini M, Gerbella M, Rozzi S, Luppino G (2007) A multiarchitectonic approach for the definition of functionally distinct areas and domains in the monkey frontal lobe. J Anat 211:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmalih A, Borra E, Contini M, Gerbella M, Rozzi S, Luppino G (2009) Multimodal architectonic subdivision of the rostral part (area F5) of the macaque ventral premotor cortex. J Comp Neurol 512:183–217. [DOI] [PubMed] [Google Scholar]
- Bettio F, Demelio S, Gobbetti E, Luppino G, Matelli M (2001) Interactive 3-D reconstruction and visualization of primates cerebral cortex. Soc Neurosci Abstr 728 724. [Google Scholar]
- Bonini L (2015) The extended mirror neuron network: Anatomy, origin, and functions. Neuroscientist. [DOI] [PubMed] [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G (2014) Ventral premotor neurons encoding representations of action during self and others’ inaction. Curr Biol 24:1611–1614 Available at: 10.1016/j.cub.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L (2010) Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex 20:1372–1385. [DOI] [PubMed] [Google Scholar]
- Bonini L, Serventi FU, Simone L, Rozzi S, Ferrari PF, Fogassi L (2011) Grasping neurons of monkey parietal and premotor cortices encode action goals at distinct levels of abstraction during complex action sequences. J Neurosci 31:5876–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra AE, Gerbella M, Rozzi S, Luppino G (2017) The macaque lateral grasping network: a neural substrate for generating purposeful hand actions. Neurosci Biobehav Rev 75:65–90 Available at: 10.1016/j.neubiorev.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G (2008) Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex 18:1094–1111. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Gerbella M, Rozzi S, Luppino G (2010) Projections of the hand field of the macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol 518:2570–2591. [DOI] [PubMed] [Google Scholar]
- Borra E, Gerbella M, Rozzi S, Luppino G (2011) Anatomical evidence for the involvement of the macaque ventrolateral prefrontal area 12r in controlling goal-directed actions. J Neurosci 31:12351–12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni S, Giorgetti V, Bonini L, Fogassi L (2015) Processing and Integration of Contextual Information in Monkey Ventrolateral Prefrontal Neurons during Selection and Execution of Goal-Directed Manipulative Actions. J Neurosci 35:11877–11890 Available at: http://www.jneurosci.org/content/35/34/11877.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano V, Fogassi L, Rizzolatti G, Thier P, Casile A (2009) Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science 324:403–406. [DOI] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C (2012) Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J Neurosci 32:3791–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL (1995) Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363:642–664. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL (2003) Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A 100:5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana F, Avanzini P, Gozzo F, Pelliccia V, Casaceli G, Rizzolatti G (2016) A mirror mechanism for smiling in the anterior cingulate cortex. Emotion:187–190. [DOI] [PubMed] [Google Scholar]
- Caruana F, Gallese V (2012) Overcoming the emotion experience/expression dichotomy. Behav Brain Sci 35:145–146. [DOI] [PubMed] [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V (2011) Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol 21:195–199 Available at: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Casile A, Caggiano V, Ferrari PF (2011) The mirror neuron system: a fresh view. Neuroscientist 17:524–538 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3743423&tool=pmcentrez&rendertype=abstract [Accessed November 3, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013) Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb Cortex 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML (2015) Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci 112:201514761 Available at: http://www.pnas.org/cgi/content/long/112/52/16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN (1999) Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol. [PubMed] [Google Scholar]
- Cisek P, Kalaska JF (2004) Neural correlates of mental rehearsal in dorsal premotor cortex. Nature 431:993–996 Available at: http://www.ncbi.nlm.nih.gov/pubmed/15496925. [DOI] [PubMed] [Google Scholar]
- Contini M, Baccarini M, Borra E, Gerbella M, Rozzi S, Luppino G (2010) Thalamic projections to the macaque caudal ventrolateral prefrontal areas 45A and 45B. Eur J Neurosci 32:1337–1353. [DOI] [PubMed] [Google Scholar]
- Cook R, Bird G, Catmur C, Press C, Heyes C (2014) Mirror neurons: From origin to function. Behav Brain Sci 37:177–192. [DOI] [PubMed] [Google Scholar]
- Coudé G, Ferrari PF, Rodà F, Maranesi M, Borelli E, Veroni V, Monti F, Rozzi S, Fogassi L (2011) Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS One 6:e26822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudé G, Festante F, Cilia A, Loiacono V, Bimbi M, Fogassi L, Ferrari PF (2016) Mirror neurons of ventral premotor cortex are modulated by social cues provided by others ‘ gaze. J Neurosci 36:3145–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Manera V, Keysers C (2009) Programmed to learn? The ontogeny of mirror neurons. Dev Sci 12:350–363 Available at: http://www.ncbi.nlm.nih.gov/pubmed/19143807 [Accessed September 9, 2014]. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992) Understanding motor events: a neurophysiological study. Exp brain Res 91:176–180. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L (2003) Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci 17:1703–1714 Available at: http://doi.wiley.com/10.1046/j.1460–9568.2003.02601.x [Accessed June 27, 2016]. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L (2005) Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci 17:212–226. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Tramacere A, Simpson EA, Iriki A (2013) Mirror neurons through the lens of epigenetics. Trends Cogn Sci 17:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS (2004) Receptive Field Properties of the Macaque Second Somatosensory Cortex Evidence for Multiple Functional Representations.pdf. 24:11193–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G (2005) Parietal lobe: from action organization to intention understanding. Science 308:662–667. [DOI] [PubMed] [Google Scholar]
- Fried I, MacDonald K, Wilson C (1997) Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 18:753–765. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action recognition in the premotor cortex. Brain 119 ( Pt 2:593–609. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G (2002) Action representation and the inferior parietal lobule. Common Mech Percept Action 19:334–355. [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G (1988) Functional organization of inferior area 6 in the macaque monkey - I. Somatotopy and the control of proximal movements. Exp Brain Res 71:475–490. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Pochon J-B, Poline J-B, Tremblay L, Van de Moortele P-F, Levy R, Dubois B, Le Bihan D, Lehéricy S (2004) Distinct striatal regions support movement selection, preparation and execution. Neuroreport 15:2327–2331. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Baccarini M, Borra E, Rozzi S, Luppino G (2014) Amygdalar connections of the macaque areas 45A and 45B. Brain Struct Funct 219:831–842. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G (2010) Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb Cortex 20:141–168. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G (2011) Cortical connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct Funct 216:43–65. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Borra E, Rozzi S, Luppino G (2016) Connections of the macaque Granular Frontal Opercular (GrFO) area: a possible neural substrate for the contribution of limbic inputs for controlling hand and face/mouth actions. Brain Struct Funct 221:59–78. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Borra E, Tonelli S, Rozzi S, Luppino G (2013) Connectional heterogeneity of the ventral part of the macaque area 46. Cereb Cortex 23:967–987. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H, Kaas JH (2011) Multiple Parietal–Frontal Pathways Mediate Grasping in Macaque Monkeys. J Neurosci 31:11660–11677 Available at: http://www.jneurosci.org/content/31/32/11660.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, Duin B van, Burga H van der (1995) Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res 23:269–279. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG (2007) Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol 97:1671–1683. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Borra E, Matelli M, Luppino G (2006) Architectonic organization of the inferior parietal convexity of the macaque monkey. J Comp Neurol 496:422–451. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim K-S, Mailly P, Calzavara R (2006) Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. J Neurosci 26:8368–8376 Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0271–06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Nieder A (2013) Single neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nat Commun 4:2409 Available at: http://www.ncbi.nlm.nih.gov/pubmed/24008252. [DOI] [PubMed] [Google Scholar]
- Hast MH, Fischer JM, Wetzel AB, Thompson VE (1974) Cortical motor representation of the laryngeal muscles in Macaca mulatta. Brain Res 73:229–240. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML (2010) Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci 4:223 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3001991&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Husler EJ, Maier MA, Qi HX (1994) Force related neuronal activity in two regions of the primate ventral premotor cortex. Can J Physiol Pharmacol. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J (1998) Task-dependent selectivity of movement-related neuronal activity in the primate prefrontal cortex. J Neurophysiol 80:3392–3397. [DOI] [PubMed] [Google Scholar]
- Huang CS, Hiraba H, Sessle BJ (1989) Input-output relationships of the primary face motor cortex in the monkey (Macaca fascicularis). J Neurophysiol. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J (1981) Regional distribution of functions in parietal association area 7 of the monkey. Brain Res 206:287–303. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G (2012) Functional organization of the insula and inner perisylvian regions. Proc Natl Acad Sci U S A 109:10077–10082 Available at: http://www.ncbi.nlm.nih.gov/pubmed/22647599%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3382514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzini A, Rozzi S, Borra E, Gallese V, Caruana F, Gerbella M (2015) A shared neural network for emotional expression and perception: an anatomical study in the macaque monkey. Front Behav Neurosci 9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U (2002) Neural pathways underlying vocal control. Neurosci Biobehav Rev 26:235–258. [DOI] [PubMed] [Google Scholar]
- Jürgens U (2009) The Neural Control of Vocalization in Mammals: A Review. J Voice 23:1–10. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Ehrenreich L (2007) The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Res 1148:90–95. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN (2009) Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron 64:922–930 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2862290&tool=pmcentrez&rendertype=abstract [Accessed September 1, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M (1995) A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15:3821–3839 Available at: http://www.ncbi.nlm.nih.gov/pubmed/7751949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle HE 15:185–234 (1978) An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (area 6 and 9) in Macaca fascicularis. Brain Behav Evol 15: 185–234. [DOI] [PubMed] [Google Scholar]
- Kuraoka K, Konoike N, Nakamura K (2015) Functional differences in face processing between the amygdala and ventrolateral prefrontal cortex in monkeys. Neuroscience 304:71–80. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tanji J (1986) Premotor cortex neurons in macaques: activity before distal and proximal forelimb movements. J Neurosci 6:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CR (1991) On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain Res 552:77–86. [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Moran R, Ungerleider LG, Ishai A (2016) Facial Expressions Evoke Differential Neural Coupling in Macaques. Cereb cortex (New York, NY 1991):bhv345 Available at: http://cercor.oxfordjournals.org/content/early/2016/01/11/cercor.bhv345.full%5Cnpapers3://publication/doi/10.1093/cercor/bhv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G (1991) Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J Comp Neurol. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rozzi S, Calzavara R, Matelli M (2003) Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci 17:559–578. [DOI] [PubMed] [Google Scholar]
- Maeda K, Ishida H, Nakajima K, Inase M, Murata A (2015) Functional properties of parietal hand manipulation-related neurons and mirror neurons responding to vision of own hand action. J Cogn Neurosci 27:560–572. [DOI] [PubMed] [Google Scholar]
- Maier MA, Kirkwood PA, Brochier T, Lemon RN (2013) Responses of single corticospinal neurons to intracortical stimulation of primary motor and premotor cortex in the anesthetized macaque monkey. J Neurophysiol 109:2982–2998 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3680818&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranesi M, Livi A, Bonini L (2015) Processing of Own Hand Visual Feedback during Object Grasping in Ventral Premotor Mirror Neurons. J Neurosci 35:11824–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranesi M, Rodà F, Bonini L, Rozzi S, Ferrari PF, Fogassi L, Coudé G (2012) Anatomo-functional organization of the ventral primary motor and premotor cortex in the macaque monkey. Eur J Neurosci 36:3376–3387. [DOI] [PubMed] [Google Scholar]
- Martin RE, Sessle BJ (1993) The role of the cerebral cortex in swallowing. Dysphagia 8:195–202 Available at: http://www.ncbi.nlm.nih.gov/pubmed/8359039. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G (1986) Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol 251:281–298. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G (1996) Thalamic input to mesial and superior area 6 in the macaque monkey. J Comp Neurol 372:59–87. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Fogassi L, Rizzolatti G (1989) Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol 280:468–488. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1985) Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res 18:125–136. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1991) Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311:445–462. [DOI] [PubMed] [Google Scholar]
- Mesulam M (1982) Principles of horseradish peroxidase neuro- histochemistry and their applications for tracing neural pathways In: Tracing neural connections with horseradish peroxidase., Wiley; (Mesulam MM, ed), pp 1–152. Chichester (UK). [Google Scholar]
- Minxha J, Mosher C, Morrow JK, Mamelak AN, Adolphs R, Gothard KM, Rutishauser U (2017) Fixations Gate Species-Specific Responses to Free Viewing of Faces in the Human and Macaque Amygdala. Cell Rep 18:878–891 Available at: http://linkinghub.elsevier.com/retrieve/pii/S2211124716318058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitz AR, Wise SP (1997) The Somatotopic Organization of the Supplementary Motor Area: lntracortical Microstimulation Mapping. J Neurosci 7:101–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS (2001) Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain 124:176–208. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Mcneal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW (2007) Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol 500:134–165. [DOI] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM (2014) Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Curr Biol 24:2459–2464 Available at: 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G, Keysers C, Gazzola V, PTRS B, Cattaneo L, Fabbri-destro M, Rozzi S, Soekadar SR, Witkowski M, Birbaumer N, Cohen LG (1997) Object Representation in the Ventral Premotor Cortex ( Area F5 ) of the Monkey. J Neurophysiol 78:2226–2230. [DOI] [PubMed] [Google Scholar]
- Murray L, De Pascalis L, Bozicevic L, Hawkins L, Sclafani V, Ferrari PF (2016) The functional architecture of mother-infant communication, and the development of infant social expressiveness in the first two months. Sci Rep 6:39019 Available at: http://www.nature.com/articles/srep39019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Borra E, Gerbella M, Rozzi S, Luppino G, Vanduffel W, Rizzolatti G, Orban G a (2011) Action observation circuits in the macaque monkey cortex. J Neurosci 31:3743–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban G a (2005) Observing others: multiple action representation in the frontal lobe. Science 310:332–336. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W (2011) Grasping-related functional magnetic resonance imaging brain responses in the macaque monkey. J Neurosci 31:8220–8229 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3117146&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Scalaidhe SP, Wilson FA, Goldman-Rakic PS (1997) Areal segregation of face-processing neurons in prefrontal cortex. Science 278:1135–1138. [DOI] [PubMed] [Google Scholar]
- Ó Scalaidhe SP, Wilson FAW, Goldman-Rakic PS (1999) Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: Evidence for intrinsic specialization of neuronal coding. Cereb Cortex 9:459–475. [DOI] [PubMed] [Google Scholar]
- Ogawa H (1994) Gustatory cortex of primates: anatomy and physiology. Neurosci Res 20:1–13. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ito SI, Nomura T (1989) Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci Res 6:283–298. [DOI] [PubMed] [Google Scholar]
- Olszewski J (1952) The thalamus of the macaca mulatta. Basel: Karger. [Google Scholar]
- Petrides M, Pandya DN (2002) Association pathways of the prefrontal cortex and functional observations. London: Oxford University Press. London: Oxford University Press. [Google Scholar]
- Raos V, Umiltá M- A, Murata A, Fogassi L, Gallese V (2006) Functional Properties of Grasping-Related Neurons in the Ventral Premotor Area F5 of the Macaque Monkey. J Neurophysiol 95:709–729. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988) Functional organization of inferior area 6 in the macaque monkey - II. Area F5 and the control of distal movements. Exp Brain Res 71:491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S (2014) Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev 94:655–706. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2:661–670. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001) The cortical motor system. Neuron 31:889–901. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C (2016) The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci 17:757–765 Available at: http://www.nature.com/doifinder/10.1038/nrn.2016.135%5Cn http://www.ncbi.nlm.nih.gov/pubmed/27761004. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Diehl MM (2011) Neurons responsive to face-view in the primate ventrolateral prefrontal cortex. Neuroscience 189:223–235 Available at: 10.1016/j.neuroscience.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G (2006) Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex 16:1389–1417. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Coudé G (2015) Grasping actions and social interaction: neural bases and anatomical circuitry in the monkey. Front Psychol 6:973 Available at: http://www.ncbi.nlm.nih.gov/pubmed/26236258%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4500865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L (2008) Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci 28:1569–1588. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S (2011) Cognitive and Perceptual Functions of the Visual Thalamus. Neuron 71:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y, Ishida F, Shimizu T, Murata A (2010) Time course of information representation of macaque AIP neurons in hand manipulation task revealed by information analysis. J Neurophysiol 104:3625–3643 Available at: http://www.ncbi.nlm.nih.gov/pubmed/20943943. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R (1992) Role of primate basal ganglia and frontal cortex in the internal generation of movements - I. Preparatory activity in the anterior striatum. Exp Brain Res 91:363–384. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone L, Bimbi M, Rodà F, Fogassi L, Rozzi S (2017) Action observation activates neurons of the monkey ventrolateral prefrontal cortex. Sci Rep 7:44378 Available at: http://www.nature.com/articles/srep44378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone L, Rozzi S, Bimbi M, Fogassi L (2015) Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal cortex. Eur J Neurosci 42:2882–2894. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U (2002) Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res 949:23–31. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U (2003) Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res 974:43–59. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Dohery J, Kaube H, Dolan RJ, Frith CD (2004) Empathy for pain involves the affective but not sensory components of pain. Science (80- ) 303:1157–1162 Available at: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Diltz MD, Averbeck BB, Romanski LM (2006) Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci 26:11138–11147 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2767253&tool=pmcentrez&rendertype=abstract%5Cn http://www.ncbi.nlm.nih.gov/pubmed/17065454%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2767253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazumi T, Hori E, Maior RS, Ono T, Nishijo H (2010) Neural correlates to seen gaze-direction and head orientation in the macaque monkey amygdala. Neuroscience 169:287–301. [DOI] [PubMed] [Google Scholar]
- Tramacere A, Ferrari PF (2016) Faces in the mirror, from the neuroscience of mimicry to the emergence of mentalizing. J Anthropol Sci 94:113–126. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J (2015) Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Mov Disord. [DOI] [PubMed] [Google Scholar]
- Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73:59–86. [Google Scholar]
- West R a Larson CR (1995) Neurons of the anterior mesial cortex related to faciovocal activity in the awake monkey. J Neurophysiol 74:18561869. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G (2003) Both of Us Disgusted in My Insula. Neuron 40:655–664 Available at: http://www.sciencedirect.com/science/article/pii/S0896627303006792. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Saito N, Iriki A, Isoda M (2011) Representation of others’ action by neurons in monkey medial frontal cortex. Curr Biol 21:249–253. [DOI] [PubMed] [Google Scholar]


